Lupine Publishers | Journal of Research & Review on Health Care
Abstract
Background: The study evaluated nano structured extract Morinda citrifolia L. (Noni) conveyed in enteral form, in an experimental model of intestinal ischemia/reperfusion (I/R), as well as in the prevention of bacterial translocation.
Purpose: Observe the protective and repairing effect of Noni’s nanoemulsified extract in the presence of induced bacterial translocation, by an experimental model of intestinal ischemia/reperfusion.
Methods and Findings: The study consisted of 2 groups of 7 animals, where the Controls were treated with 0.9% saline solution (I/R + S) and Experimental group (I/R + Noni) treated with 5 mg/ml/Kg Noni nanoemulsion, orally by gavage 12h and 2h before the experiment. In the groups, the superior mesenteric artery was occluded with a vascular micro clamp and the laparotomy reopened 60min. after for pull back the clamp. Treatment response was assessed by blood count, inflammatory markers and biochemical dosages, including a sample of the terminal ileum and hepatic of each animal was harvested, fixed in formalin 10% and included in paraffin and stained with hematoxylin-eosin for morphometric measurement. Measurement of TNF-α, IL-1β, IL-6, and IL-10 was done. One gram of spleen, liver, and mesenteric lymph nodes were harvested for culture by selective means for Gram (-) and Gram (+) bacteria. ANOVA and the post-hoc Turkey and Student´s t test were used, considering p<0, 05 as significant.
Conclusion: Noni´s nanoemulsion positively influenced the organic reactions in the presence of intestinal ischemia/reperfusion, reducing the production of pro inflammatory cytokines, bacterial translocation, preventing tissue injury and attenuating the systemic inflammatory response against the experimental model used
Keywords: Noni: Ischemia-reperfusion injury; Bacterial translocation; Biotechnology; Nanotechnology; phytotherapy
Abbreviations: GGT: Gamma-glutamyl Transferase; ECUA: Ethics Committee on the Use of Animals; NCCAE: National Council for Control of Animal Experimentation; NO: Nitric oxide; AST: glutamic-oxalacetic transaminase; ALT: glutamic-pyruvic transaminase; HSV: total bilirubin and fractions, erythrocyte sedimentation rate; SMA: Superior Mesenteric Artery
Mammography
Intestinal ischemia-reperfusion (I-R) injury is a severe condition resulting from acute mesenteric ischemia, small bowel transplantation, abdominal aortic aneurysm, hemorrhage, trauma, septic shock, or severe burns. Various chemical and cellular mediators have been implicated in the pathogenesis of intestinal ischemia/reperfusion, such as reactive oxygen, cytokines, endotoxins, and neutrophils [1]. Following adhesive interactions among neutrophils and endothelial cells, neutrophil accumulation in the intestinal mucosa contributes to intestinal ischemia/ reperfusion injury via production of reactive oxygen metabolites and proteases. Leukocyte accumulation is a complex phenomenon that also involves endothelium-based adhesion molecules as well as leukocyte chemotaxis factors such as interleukin-8 (IL-8) [2,3]. Intercellular adhesion molecules are normally expressed at a low basal level, but their expression can be enhanced by several inflammatory cytokines such as IL-1β and tumour necrosis factor-α (TNF-α). A variety of cytokines, including TNF-α, interferon-γ, and IL-1β, are released from post-ischemic tissues [4].
It is now considered that these mediators bring about the systemic microcirculatory injury which is thought to be the main mechanism responsible for damage in sepsis. The host defence responses to sepsis may promote the generalized increase in leukocyte recruitment and accumulation in the tissues, which may lead to subsequent endothelial damage, leaky capillaries, and organ dysfunction and failure [5,6]. The organic lesion begins in the lungs, progressing to the anguish respiratory syndrome, followed by kidney and liver failure because of damage caused by the architecture of these organs. Heart failure occurs in late-stage sepsis [7]. The pathophysiology of intestinal ischemia/reperfusion (I/R) in rats with induction of bacterial translocation is widely used in animal models and well established in the literature, and is a viable way of analyzing therapeutic options [8].
The technique will result in a bacterial translocation, generating an ideal model for the therapeutic trial with Noni. Medicinal plants influence the health conditions of the people, in part due to the increase of studies with phytotherapeutics, leading to a confirmation of the therapeutic action of several popular plants, a fact that proves Phytotherapy as part of the culture of the population, being used and widespread for many generations[9-11]. In Brazil, the use of medicinal herbs has its bases in the indigenous practice, which influenced by the African and Portuguese culture, generated a vast popular culture. With the technological advances in allopathic medicine and the pharmaceutical industry in recent years, herbal medicines have been placed in the background, being something allied to popular belief and without scientific bases [12]. However, due to side effects and the high cost of medicines, Phytotherapy is again highlighted and scientific studies with medicinal plants are being resumed [13].
Among the species for herbal treatment highlights the Morinda citrifolia L. (Rubiaceae), popularly known as Noni. Information on its therapeutic benefits has gone through the world causing great demand as a medicinal product [14-16]. Morinda citrifolia L (Noni) has the phytotherapeutic activity of analgesic, antimicrobial, antitumor, anti-inflammatory and antioxidant effects. In scientific studies conducted for the isolation of fixed compounds of the plant, about 200 active substances have already been isolated, where the presence of anthraquinones, triterpenes, iridoids, among others [7- 9]. The anti-inflammatory activity of Noni has been studied in vivo and in vitro by inhibiting the activity of COX-1 enzymes and COX- 2, and the release of chemical mediators from macrophages (nitric oxide (NO) and prostaglandin E2 - PGE-2)[10].
In this sense, products using biotechnology in the form of nanoparticles or nanostructured compounds, can have excellent results, since, due to their reduced diameter, the substance can be used in smaller doses, avoiding the toxic effect of the plant and maintaining its phytotherapic action [11-13]. Thus, the objective of the present study was to evaluate the protective and repairing effect of Noni’s nanoemulsified extract in the presence of induced bacterial translocation, by an experimental model of intestinal ischemia/ reperfusion (I/R) in rats, using the dosage and subsequent blood count, inflammatory markers and biochemical measurements, including histopathological analysis of the compared to the control group treated with 0.9% saline solution.
Material and Methods
Ethical Principles
The experimental protocol was approved by the Ethics Committee on the Use of Animals - ECUA, number 012/2016, Brazil. Animals were handled in accordance with the Guide for the Care and Use of Laboratory Animals, US National Research Council, 1996. The Institutional Committee on Ethics in the Use of Animals approved the research project under the protocol. The care with the use of the animals followed the standards of the Brazilian legislation for the scientific use of animals (Law 11.794 / 2008 National Council for Control of Animal Experimentation - NCCAE / Brazilian Government).
Preparation of vegetable extract hydroalcoholic extract of Morinda citrifolia L: (Noni)
The hydroalcoholic extract of Morinda citrifolia L. was prepared from the aerial parts (stem, leaves, and fruits) of fresh adult plants. The collected material was placed for drying at room temperature; then comminuted with a knife and placed for 24h in an oven at a temperature between 45ºC and 50ºC to remove moisture. It was then subjected to a grinding process to obtain the powder. This material was weighed and deposited in a glass vessel with the addition of 70% hydroalcoholic solution in the ratio of 1:3 of the powder. The resulting mixture was stirred for 12h and stirred for five minutes every two hours under two simple filtration procedures under reduced pressure to give the crude extract, which was concentrated in a rotary evaporator under reduced pressure, at a temperature between 55°C and 60°C, for total solvent elimination. The product obtained after concentration was in the form of a paste which was diluted in distilled water until a hydroalcoholic extract at the concentration of 5mg/ml was obtained, being kept in a refrigerator at 10ºC until it was used.
Obtaining Nanoemulsified Systems
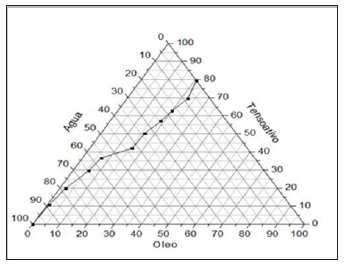
Nanoemulsion systems were obtained by maceration in a mixture of ethanol: water (8:2/800mL of alcohol 70° INPM + 200mL of distilled water) according to the previously described methodology. The study of phase diagrams of the oil, water and surfactant mixture formed a nanoemulsified (SN) system with a self-emulsifying characteristic. The SN system was considered O/W of the autoemulsifying type due to the unchanging of its appearance after successive dilutions in water. The vegetable oil used contains the following chemical components: vitamin E, oleic, linoleic and linolenic acid, low saturated fat and high content of polyunsaturated components [17,18]. The procedure used to obtain the nano emulsion regions is based on the method involving the determination of the maximum solubility points of active matter (surfactant) in the aqueous and oily phases, by means of mass titrations. The structure is composed of vegetable oil in the range of 0.5% to 6.0%; a surfactant in the range of 8.0% to 20% and distilled water in the range of 75% to 90% (Figure 1).
Figure 1: Phase Diagram (Maciel 2009).
Description of Nanoemulsion Preparation: Initially, industrialized vegetable oil was weighed on a precision digital scale (model: FA-2104N / 2008; Brand: BIOPRECISA-->) in the preferred range (1% to 5%), the surfactant in the preferred range (8% to 20%), and distilled water in the preferred range (75% to 90%) mixed and heated the components of the formulated SME - Nanoemulsion System, in magnetic stirrer with heating - stirring range 100-2200rpm and temperature controlled in the preferred range between 40°C to 70°C (Model NI1103P/2009: Mark: NOVAINSTRUMENTS-->). Thereafter, centrifuged at a constant speed, between 550rpm and 650rpm, for a preferential time of 15 minutes. (Model TDL80-2B, Mark Centribio-->).
Analysis Characterization: Performed through the refractive index, droplet diameter, rheological behavior and surface tension. To obtain the refractive index, an analog bench refractometer (ABBE, Model: 2waj; Mark: BioBrix-->) was used at a temperature in the range of 20°C-30°C.
Determination of droplet diameter: The diameter of the nanoemulsion droplets was determined by measurements in triplicates and with the refractive index of 1.4715 with a light beam of 659nm wavelength and angle of incidence of 90°. The diameter of the droplets (ranged from 50nm-75nm).
Rheology: The evaluation made from the graph generated by shear stress versus shear rate using a greenhouse thermometer (HG - Brazil, No.1876/11-->), in the range of 20°C-30°C, with variation for the shear rate (1s-1-1000s-1), resulted in a linear behaviour, with r2 = 0.91058.
Fluid Classification: The fluid under analysis was classified as Newtonian and the viscosity was determined to be 2mPa.s - 6mPa.s (2cP - 5cP).
Maximum Dilution Analysis: The evaluation was performed by experimental data of surface tension. Assays were performed using the SensaDyne Tensiometer apparatus (model QC-600, Chem-Dyne Research Corp. - USA). The description of the method consisted of measuring the maximum bubble pressure using two capillaries with holes of different diameters, where an inert gas (N2) was pumped at a certain constant pressure (200-595kPa). The larger capillary measured the effect of the immersion depth and the smaller the surface tension. For each measurement, the samples were diluted in distilled water, with concentrations varying until the values of surface tension close to the water (γH2O = 72.1mN/m) and temperature in the range of 20°C to 30°C were reached.
Surgical model / experimental design: We used 14 Wistar male Wistar rats weighing 265±32g (from Nucleus of Experimental Surgery, Potigura Universty - LAUREATE INTERNATIONAL UNIVERSITIES - UnP/Natal/Rio Grande do Norte State/ Brazil), randomly divided into 2 groups, kept in individual cages with food and water standard (Labina-Purina-->) is rodents, ad libitum. In the experimental group (n=7) rats received 5mg/mL/Kg (EMS-FC5) Noni nanoemulsion via the probe/gavage 18 and 2 hours prior to clamping of the superior mesenteric artery (I/R), the remainder rats (n=7) were treated with oral saline solution at 0.9%, 18 and 2h prior to (I/R). After 12h of fasting, the rats were anesthetized with for the induction of anesthesia general, the solution of Zoletil--> 50, the anesthetic dissociative, will be used in the dose of 0.3mL/100mg intramuscularly in the region of the quadriceps, with disposable syringes of 1mL of insulin and needle 27F and operated under aseptic conditions. In groups I/R + Saline and I/R + Noni, under sterile conditions.
Measurement of bacterial translocation: After shaving, the abdominal skin was disinfected with 0.2% chlorhexidine. All procedures were performed under sterile conditions. A laparotomy was performed and the superior mesenteric artery (SMA) was occluded with a microvascular clamp for 60 minutes. In order to block any collateral blood supply, the right colic and proximal jejunal arteries were also clamped. The laparotomy incision was then closed, to be opened later for removal of the clamps after 60 minutes of ischemia. Reperfusion was confirmed by the return of the mesenteric arcade pulsation. The incision was closed again and the animals were killed by anesthetic overdose (thiopental 100mg/ Kg) after 120 minutes of reperfusion. They breathed spontaneously throughout the procedures.
Measurement of bacterial translocation: At the end of the procedures (time = 180 minutes), a midline laparotomy was performed under aseptic conditions and biopsies were aseptically obtained for bacterial colony counts. One gram of mesenteric lymph node complex, blood, liver, and lung were removed for culture. Tissues were homogenized and aseptically solubilized after addition of 0.5mL of 0.9% saline. Aliquots of 0.2mL were processed and cultured on selective MacConkey’s agar and Blood Agar for detection of gram-negative and gram-positive bacteria, respectively. The agar plates were incubated at 37oC and examined for growth after 24 and 48h. Any growth in the plates of bacteria of the same biotype as cultured was considered positive and expressed as colony-forming units per gram of tissue (CFU/g).
Laboratory analysis/ Cytokine Assays: After 24h of a conclusion of the procedures under anesthesia and aseptic conditions, blood was collected by cardiac puncture to measure the hemogram, hepatogram, cytokines, and albumin. Samples of blood were treated with EDTA and the plasma was separated by centrifugation at 2000rpm and stored in -80°C for later measurement of tumor necrosis factor (TNF-a) interleukin-6 (IL-6) and interleukin- 1b (IL-1b) by the ELISA (enzyme-linked all immunoassay kits from PeproTech--> (Rocky Hill, NJ, USA) according to the manufacturer’s recommended protocols. The fluorescence was measured by a Bio-Tec--> Instruments EL808 ultra microplate reader, using KC4-V3.0 analysis software. The sensitivity of detection was 30pg/mL for all cytokines. For counting leukocytes and red cells using an automated cell counter (Abbott Cell-Dyn 3500R CD--> 5L-3500, USA). For albumin, alkaline phosphatase, gamma-glutamyl transferase (GGT), glutamic-oxalacetic transaminase (AST), glutamic-pyruvic transaminase (ALT), total bilirubin and fractions, erythrocyte sedimentation rate (HSV) blood was treated with EDTA.
Histological study: Terminal ileum and liver specimens were fixed in 10% buffered formalin and embedded in paraffin. Sections cut at a thickness of 4μm were stained with hematoxylin and eosin for morphometric measurements using an image analyzer (Image- Pro Plus, Media Cyber-->)[19-21]. The damage of the intestinal specimens was assessed in a blinded manner by an experienced pathologist according to microscopic criteria for degree of damage based on a grading system previously described: normal mucosa, 0; subepithelial space at the villus tip, 1; more extended subepithelial space, 2; epithelial lifting along villus, 3; denuded villi, 4; loss of villus tissue, 5; crypt layer infarction, 6; transmucosal infarction, 7; transmural infarction, 8.
Statistics: Data analysis was performed using the BioEstat--> 2.0 program. Differences between the microbiological samples as measured by positive cultures were evaluated by a test for differences between proportions. The results were tabulated and compared by ANOVA using post hoc analysis with Tukey and Student’s t test. P<0.05 was considered significant. Data on continuous quantitative variables are the mean ± expresso standard deviation. In the variables that did not present normal distribution, the logarithmtransformation method was adopted. These variables are represented by their respective logarithms. To verify if the differences between the Experimental (Noni nanoemulsion) and Control groups were statistically significant, the Student’s t-test for independent samples was used. The statistical package SPSS-->21 was used.
Results
We observed bacterial translocation to mesenteric lymph nodes, liver, lung, and blood in all animals subjected to I / R. However, in I/R group rats treated with Noni nanoemulsion, translocation to these organs and blood was significantly lower than in I/R untreated (Table 1). Cytokines had lower levels of proinflammatory cytokines in group I/R+Noni (TNF-α, IL-1β, IL-6) and a higher level of antiinflammatory-cytokine (IL-10), when compared with I/R + Saline (C) (Table 2). In I/R + Saline (Control) group rats, the levels of pro-inflammatory cytokines were significantly higher when compared to I/R+Noni. This group had the highest values of IL-10 when compared with (Control) group (p<0.05). Noni was able to maintain and modulate the inflammatory reaction in the experimental group, which was proven through normality in the hematological dosages. There was also a significant reduction in the number of total leukocytes, which did not generate immune suppression in experimental animals, but control the systemic inflammatory response in the presence of induced bacterial translocation for intestinal ischemia and reperfusion.
Table 1: Bacterial Translocation in groups treated and not treated with Noni nanoemulsion (colony-forming units per gram of tissue - CFU/g).
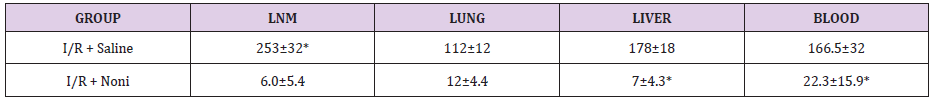
*p < 0.01 compared with groups I/R + Saline (C) and I/R + Noni nanoemulsion.
Table 2: Serum levels of cytokines in groups with and without Noni nanoemulsion treatment.
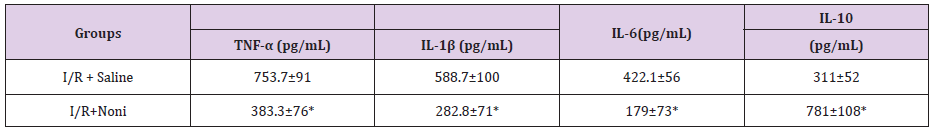
*p< 0.01 compared with groups I/R + Saline (C) and I/R + Noni nanoemulsion.
Table 3: Descriptive and inferential statistics of hemograma results.
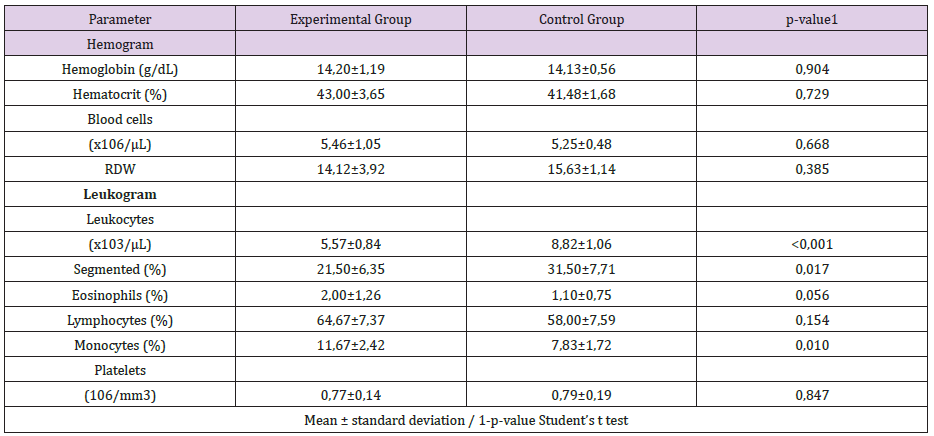
Figure 2: The mucosa is injured, and leukocyte infiltration of lamina propria and mucosa are shown (group I/R), 100x. 2: Hemorrhage and inflammation of mucosa (group I/R), 100x.
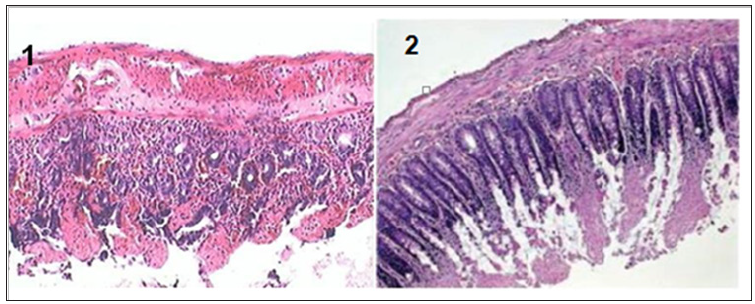
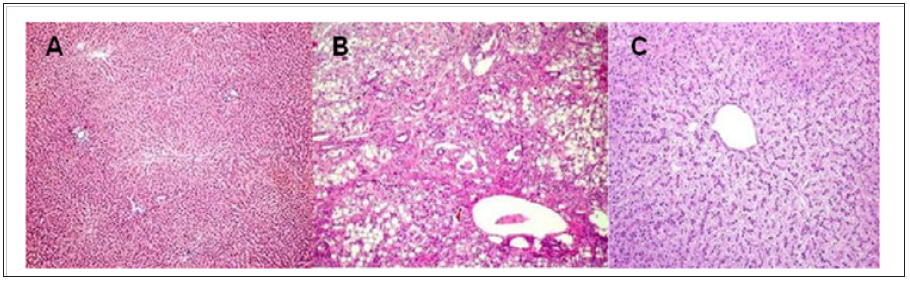
A trend towards normality was observed in the other cellular parameters measured in relation to the control group (Table 3). Macroscopically, the segments of the organs studied presented intramural dilation and hemorrhage, with greater intensity in rats of the I / R group, compared to the experimental group (Noni). Microscopic findings revealed marked mucosal lesion after ischemia and reperfusion injury; we observed more intense lesions in the rats of the I / R group compared to the other groups. The most frequent lesions were: disorganization of the normal tissue structure, transmural infarction, infiltration of leukocytes in the lamina propria and mucosa, alveoli and hepatic sinusoids. In the group that used the nanoemulsion of Noni, there was protection and preservation of the tissue structure, with reduced or absent infiltrating inflammatory reaction (Figures 2-4).
Figure 3: Preserved intestinal mucosa, demonstrating normal villi and intestinal epitelial cells of uniform pattern, normal to histological examination, 100x
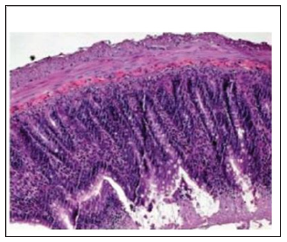
Figure 4: A) Normal liver structures are demonstrated. B) A hepatic section from a septic rat with vehicle (normal saline) treatment. Patches of hepatocytes show necrosis with eosinophilic cytoplasm nuclei that are condensed and intensely stained with hematoxylin. C: A section from a septic rat with Morinda citrifolia L (Noni) treatment. Liver structures appear normal. (HE.50;100 x /100 μm).
Discussion
The present study used an experimental model of ischemia and reperfusion, to verify the effect of nanostructured extract Morinda citrifolia L. (Noni) on intestinal injury and bacterial translocation. Some authors consider the bacterial factor, crucial in the pathogenesis of sepsis and multiple organ failures [22]. In surgery and intensive care, intestinal obstruction and intestinal ischemia are the most associated pathological conditions [23]. The use of medicinal plants for the treatment of diseases has been occurring since the dawn of civilization. The development of methodologies for the isolation of active substances has made it possible to identify substances in complex samples such as plant extracts. In this way, the interest for compounds of vegetal origin that could be used as prototypes for the development of new drugs resurfaced [24]. Medicinal plants represent the main raw material used for the synthesis of medicinal products, besides being used as therapeutic agents. Plant consumption is overvalued in traditional use based on it medicinal benefits [25].
Noni (Morinda citrifolia L.) has become a promise of the cure for a variety of diseases, ranging from simple hypertension to malignant tumors; even provokes the cure of syndromes, still incurable such as AIDS and other viral diseases [26]. However, The popular use and wellbeing attributed to Noni make the industry commercially explore Morinda citrifolia L products, often without scientific evidence [15].In this context, the use of biotechnology transforming Noni into a nanostructured compound, has the advantage of using the plant’s medicinal properties, reducing its toxicity, keeping its bioactive principles beneficial to health [27,28]. Antimicrobial activity of Noni has already been reported in the literature. Studies have shown that noni inhibited growth in vivo and in-vitro bacterial strains such as Staphylococcus aureus, Pseudomonas aeruginous, Bacillus subtilis, Escherichia coli, Helicobacter pylori, Salmonella and Shigella.
In addition, Noni has already been studied on its effect against Plasmodium falciparum, believed to be due to the presence of anthraquinones, acubin, L-asperuloside, alizarin, scopoletin, among other substances [29,30]. It has also been found that ethanol and hexane extracts of noni have an antitubercular effect since they inhibit by 89-95% the growth of Mycobacterium tuberculosis. The major components identified in the hexane extract were E-phytol, cycloartenol, stigmasterol, b-sitosterol, campesta-5,7,22-trien-3-bol, and the ketosteroids, stigmasta-4-en-3-one and stigmasta-4-22- dien-3-one[18-20]. Moreover, they showed that the anti-microbial effect is highly dependent on the stage of ripeness and on processing, being greater when the fruit is ripe, without drying [23-25]. The limiting factor for the use of Noni as an herbal remedy is that most of the studies previously found in the literature, administer the extract in the alcoholic or hydro-alcoholic form, which may, through prolonged use, mainly cause hepatotoxicity [24].
With this in mind, the present study makes its scientific contribution to demonstrate that with the use of biotechnology in the formulation of a nanostructured extract of Noni, such undesirable side effects were abolished, maintaining the active principles of the vegetable under analysis [31]. Recent studies have demonstrated, respectively, the efficiency of Noni´s nanoemulsion in experimental models of healing of infected wounds in the skin of rats as well as in abdominal sepsis induced by cecum ligation and puncture [32,33]. This phenomenon was confirmed in the present study, where a reduction in the total leukocytes and polymorphonuclear levels was observed, maintaining normal hemoglobin and hematocrit levels in the experimental group, which used Noni in the presence of an experimental model of intestinal ischemia/reperfusion (I/R) in rats with induction of bacterial translocation.
Conclusion
In conclusion, the present study demonstrated that Noni’s nanoemulsified extract acted as an immune modulatory agent in the presence of an experimental model of intestinal ischemia/ reperfusion (I/R) in rats with induction of bacterial translocation, reducing the systemic inflammatory response, stimulating the immunity of experimental group animals, preserving liver function and maintaining its bioactive principles beneficial to the experimental model used.
Read More Lupine Publishers Research & Reviews Journal Please click on below link
https://lupinepublishers.blogspot.com/

No comments:
Post a Comment
Note: only a member of this blog may post a comment.