Lupine Publishers| Archives of Diabetes and Obesity Journal
Introduction
The incidence of twin epidemics, obesity and type-2 diabetes are increasing rapidly worldwide in the past two decades [1-7]. By azand large, clinicians manage diabetes by meticulous management of blood glucose levels to meet the guidelines established by professional societies and regulatory agencies (8, 9). The American College of Physicians (ACP) published the most recent guideline in April of this year (8). Major guidance statements include:
a) Clinicians should personalize goals for glycemic control in patients with type-2 diabetes on the basis of a discussion of benefits and harms of pharmacotherapy, patients’ preferences, patients’ general health and life expectancy, treatment burden, and costs of care.
b) Clinicians should aim to achieve an HBA1c level between 7% and 8% in most patients with type-2 diabetes.
c) Clinicians should consider de-intensifying pharmacologic therapy in patients with type-2 diabetes who achieve HBA1c levels less than 6.5%.
d) Clinicians should treat patients with type-2 diabetes to minimize symptoms related to hyperglycemia and avoid targeting an HBA1c level in patients with a life expectancy less than 10 years due to advance age (80 years or older), residence in a nursing home, or chronic conditions (such as dementia, cancer, heart failure) because the harms outweigh the benefits in this population [8]. Recently three major professional bodies have issued guidelines on this topic. They are, American Association of Clinical Endocrinologists, American College of Endocrinology, and the American Diabetes Association [9]. In this overview (point of view), we will briefly discus some of the recent emerging technologies that may help empower and encourage patients to monitor their glucose levels and better manage their post-meal glycemic load.
According to Rebecca Voelker’s report in the recent issue of JAMA Network (May 2018), the.com Food and Drug Administration (FDA) has approved a continuous glucose monitor (CGM) that can work in tandem with mobile medical apps and automated insulin pumps to help people with diabetes manage their interstitial sugar more easily. The Dexcom G6 is the first CGM approved as both a stand-alone device and one that can be integrated into automated insulin dosing systems. According to the manufacturer, Dexcom Inc. of San Diego, California, its newly approved CGM has an easyto- use auto applicator that inserts a small sensor just beneath the skin. The sensor measures glucose levels and a transmitter inside of it sends readings wirelessly every 5 minutes to a receiver or a compatible smartphone or smartwatch. With a mobile app, users can share readings with up to 5 people. No finger sticks are needed for calibration or diabetes treatment decisions. In 2 trials, 324 adults and children aged 2 years or older with diabetes used the Dexcom G6 for 10 days. During multiple clinic visits, their readings were compared with laboratory test results that measured their blood glucose levels. An FDA statement indicated that no adverse events were reported during the studies. We would be glad to validate these sensors if the manufacturers provide them for our clinical evaluation in India.
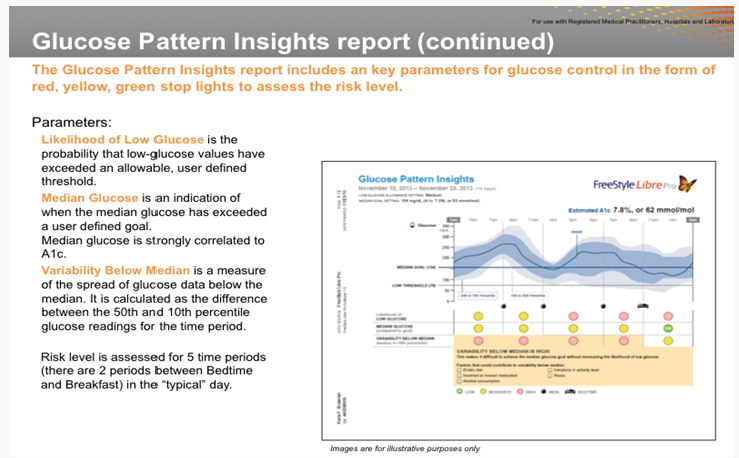
We in India are validating another CGM (Ambulatory Glucose Monitor, AGM) that is developed by Abbott Diabetes Care, Free Style Libre Pro (www.freestylelibre.com). The sensor comes with an easyto- use auto applicator. Manufacturers claim that the sensor is 48% less bulky than the Dexcom G5 transmitter and sensor. One has to use the reader that is provided by Abbott Diabetes Care. Abbott as well as iPhone do not have any apps to monitor the data from these sensors. However, android phones (with Near Frequency Communication capabilities) can read the data from these sensors. According to my collaborator Dr. Santosh, parents of type-1 diabetes patients love this system as they can keep an eye on the sugar levels of their children. To be competitive, iPhones and Abbott also should develop smart phone Apps. Abbott sensor measures glucose levels every 15 minutes for two weeks. Risk level is assessed for five time periods (there are 2 periods between bedtime and breakfast). A typical graph of median glucose is shown below. Abbott claims a strong correlation between median glucose and HBA1c (Figure 1).
We are validating the usefulness or otherwise of this new glucose sensor in two independent sites in India (All India Institute of Medical Sciences, Patna, and Karnataka Institute of Endocrinology and Research, Bengaluru). The author has done some preliminary study using this sensor and a typical summary of the results are shown below. Since this study was done by an authorized endocrinologist of Karnataka Institute of Endocrinology and Research (KIER) using me as the test subject, I will refer the subject as the patient. He is (a resident of USA) over 80 years of age with well characterized type-2 diabetes for over 20 years. He is under medications (Metform in 500mg two tablets, twice daily, DPP-4 inhibitor Januvia 50mg/twice daily, Glipizide 10Mg/twice daily) and the graph shown below shows the results of a wellmanaged glucose profile. Author thanks Dr. Santosh Olety(KIER) and MS. Smitha Raja (Noesys software, Bengaluru) for these studies and analysis of the data.
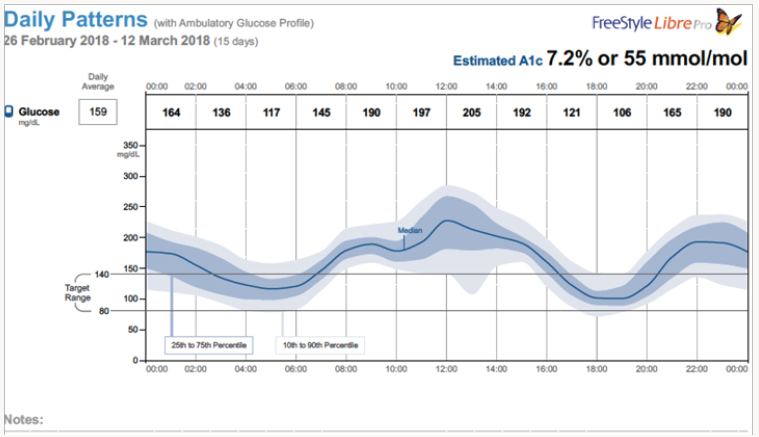
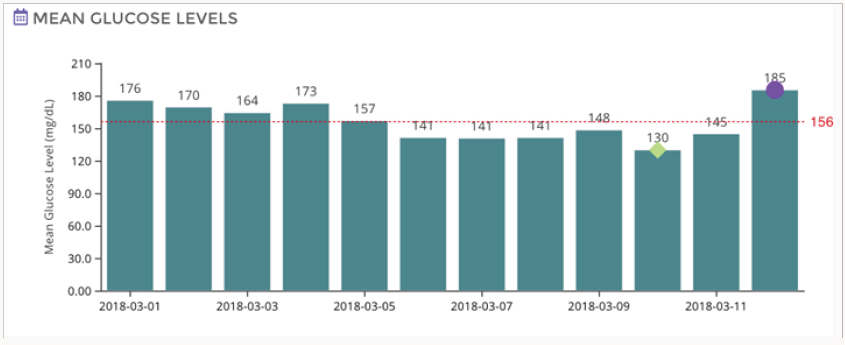
Figure 2 shows a composite data for the duration of the study (12 days). According to this data estimated HBA1c was 7.2 fairly close to the estimated clinical values. Figure 3 gives the mean glucose values for each day (variation in the mean values are not very significant). Currently, we are conducting studies to determine the correlation between the blood glucose measured by the finger prick method and the interstitial glucose measured by these emerging technologies. We also are evaluating the usefulness or otherwise of this method of glucose monitoring for the management of postmeal glucose as well as for screening of indigenous anti-diabetic drugs and their combinations.
As the manufacturers of glucose sensors (Dexcom and Abbott) claim, the new glucose sensors that can work in tandem with mobile medical apps and automated insulin pumps help people with diabetes manage their interstitial sugar more easily. As discussed in this overview, these sensors will serve as useful tools to manage post-meal glucose levels as well as for screening anti-diabetic drugs, dietary supplements and herbal products. Emerging technologies in device development, availability of mobile apps, improved analytics will play a very important role in the development of better affordable healthcare.
Acknowledgementment
Author thanks the following collaborators: Dr. M. A Shekar, The Director, Karnataka Institute of Endocrinology and Research (KIER), Dr. Santosh Olety, Pediatric Endocrinologist, KIER, Ms. Smitha (Raja) Bopanna, Co-Founder, Director, Noesys Software, Pvt, Ltd, Bengaluru. Dr. Sadhana Sharma, Professor, Head, Department of Biochemistry, All India Institute of Medical Sciences, Patna. Abbott Diabetes Care, Regional Representatives: Dr. Navneeth Selvan (Southern Region), Dr. Silpi Bardhan (Eastern Region).
Read More Lupine Publishers Archives of Diabetes and Obesity Please Click on Below Link https://diabetes-obesity-lupine-publishers.blogspot.com/

No comments:
Post a Comment
Note: only a member of this blog may post a comment.