Lupine Publishers | Journal of Organic and Inorganic Chemical Sciences
Abstract
Low cost adsorbent (activated carbon) with a high metal adsorption capacity was prepared from the disposal sewage sludge by physical activation method (Pyrolysis temperatures at 500 °C, 650 °C, 700 °C, 800 °C and 900 °C). The resulting material was evaluated as adsorbents for the removal of Cd, Pb, Cu, and Mn from aqueous solution. The results revealed that sewage sludge activated by pyrolysis at 800 °C [ASS800] was the most effective adsorbent for the removal of Cd, Pb, Cu and Mn from polluted water. Batch studies were performed to evaluate the influences of various experimental parameters such as pH, initial metal ion concentration, adsorbent dosage, and contact time and solution temperature. Adsorption of Cd, Pb, Cu and Mn by ASS800 increased with increasing pH and adsorbent dosage, while decreasing with the increase of initial metal concentration. From batch adsorption data the optimum conditions for the adsorption of Cd , Pb, Cu and Mn by ASS800 were 1gL-1 adsorbent dosage, 1 hour contact time, 10mgL-1 initial metal concentration, 25 °C solution temperature; and pH 6 . Adsorption data were well fitted to the Langmuir, and Freundlich isotherms. Thus, simple pyrolysis provide conversion of sewage sludge into inexpensive low-rank adsorbent potentially useful for the removal of heavy metals from polluted water .
Keywords: Adsorption; Heavy metals; Activated carbon; Sewage sludge; Treatment
Introduction
Sewage sludge as waste material produced from the treatment of municipal waste water. Due to their unstable nature of biomass, sewage sludge disposal may cause environmental problems, so it is important to save environment from pollution with this sewage sludge. For converting the sewage sludge to more useful material [1].
Sewage sludge contains high organic contents from 60 to 80%; therefore it is important to use the sewage sludge for the production of low-cost activated carbons [2]. Sewage sludge has received great attention in the preparation of effective adsorbents with environmental applications. Adsorbents from sewage sludge have been prepared by different methods includes chemical activation [3-6], and physical activation [7-9].
Various types of adsorbents were developed from sewage sludge. De Filippis et al. [10] studied the removal of Cu, Zn and Cd from wastewater using adsorbents produced from pyrolysis of sewage sludge at 550 °C. Rashed et al. prepared adsorbent by chemical activation of sewage sludge. [7] studied the removal of copper from synthetic wastewater by adsorption on sewage sludge adsorbent [11] prepared activated carbon from sewage sludge by the pyrolysis at 500 °C for 3h. Rashed [12] used washed sewage sludge and sludge activated carbon as adsorbents for removal of Acid Blue 93 from industrial wastewater. Hammaini et al. studied the effect of pH and biomass concentration on the biosorption of Cu, Cd, Zn , Ni , and Pb using activated sludge.
In this study, physically activated adsorbent was prepared from sewage sludge by pyrolysis it at various temperatures (500 °C, 650 °C, 700 °C, 800 °C,or 900 °C) and time. The developed suitable adsorbent was applied for adsorption and removal of Cd, Pb, Cu, and Mn ions from aqueous solutions. The removal efficiency of the developed adsorbent was investigated as a function of pH, contact time, initial metal concentration, temperature and adsorbent dose.
Material and Methods
Material, chemicals and reagents
All chemicals and reagents used were analytically grade. All the batch experiments were carried out in a Pyrex conical beaker (100ml) at room temperature under mechanical stirring (150rpm), the solution pH was adjusted with (1N) NaOH or HCl.
Sample collection
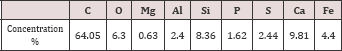
Sewage Sludge: Sewage sludge sample (5Kg) was collected from sewage sludge disposal of Kima wastewater treatment plantat Aswan city, Egypt. The sewage sludge mixed liquor was washed with distilled water, air dried in a clean place, and then oven dried at 105 °C for 24h. The resulting adsorbent was grinding and sieving within 63|im . The chemical composition of sewage sludge sample was cited in Table 1.
Table 1: Chemical composition of raw sewage sludge.
Wastewater Sample: Wastewater sample was used to evaluate the potential efficiency of the developed adsorbents for the removal of heavy metals from wastewater. Water sampler, with handle 500ml, was used for collecting wastewater from the laboratory (heavy metals analysis) of Aswan branch of the Environmental Affairs Agency. Samples were filtered by filter paper (Whatman-42), collected in 1liter polyethylene bottles and preserved in the refrigerator at the 5 °C until the experiment.
Adsorbents preparation
Pyrolyzed materials were obtained from the dried sewage sludge (100 g) by thermal treatment for 30 min in a muffle furnace at different temperatures (500 °C, 650 °C, 700 °C, 800 °C and 900 °C ) for 1h. The resulting materials, after cooling, were washed with 1M HCl solution, followed by filtration, and then washed with deionized water until the pH of leached solution was between 6-7, after then it were dried at 105 °C for 24h, crushed and sieved to 63|im. The resulted adsorbents were labeled ASS500, ASS650, ASS700, ASS800, and ASS900, respectively. A washing procedure by 1M HCl solution and deionized waterwas to remove acid-soluble inorganic matter and residual activating reagent.
Adsorption experiments
Comparative study of the developed sewage sludge adsorbents: Dosage 150mg of the prepared adsorbents (ASS500, ASS650, ASS700, ASS800, ASS900) was stirred with 50ml single metal standard solutions (Cd, Pb, Cu and Mn) for 8h. The solution pH was adjusted to pH 6. Experiments were under gone with different initial metal concentration (10, 30, 50 and 75mgL-1), with solution temperature 25 °C. After an equilibrium contact time 8 h the samples were filtered through What man filter paper No.42. The metal ion concentration in the filtrate was measured by atomic absorption spectrophotometer and the removal percent of the metal ion by each ASS was calculated.
Batch adsorption
After selecting the best adsorbent ASS800, the following batch adsorption were carried to determine the optimum condition for adsorption process. For the effect of initial metal concentration,150 mg of ASS800 adsorbent was Shacked with 50mL standard solution of single heavy metal ions (Cd, Pb, Cuand Mn) for 8h, temperature 25 °C , pH 7 for Cd, pH 6 for Pb , pH 5.2 for Cu, and pH 9 for Mn, and various initial metal concentrations 10, 30, 50 and 75 mgL-1. 1M HNO3 or 1M NaOH was used to adjust pH value. After 8h the samples were taken and filtered through Whatman filter paper No. 42. The metal ion concentration in the filtrate was measured by atomic absorption spectrophotometer and the percent removal for metal ion by each ASS was calculated. The effect of pH (2, 3, 4 ,6 ,7, 8 and 10 ), ASS adsorbent dosage (2.5, 10, 30, 50, 100, 200 mg),contact time (0.5, 1, 2, 4, 6 h )and solution temperature (25, 35, 45 and 55 °C) were carried out.
Analytical techniques
Metal ion concentrations (Cd, Pb, Cu, and Mn)were measured by atomic absorption spectrophotometer, using hollow cathode lamps of Cd, Pb, Cu, and Mn. Absorption reading was taken at 228.8 nm for cadmium, 324.8nm for copper, 283.3nm for lead, 279.5nm for manganese. Air-C2H2 gas was used as fuel gas with flow rate ranged 1.8 -2 L/min. Metal adsorbed concentration was evaluated by the difference between initial and remaining concentration in the sample.
Morphological features
The micro-morphology and chemical composition of the raw Kima sewage sludge and the developed adsorbent (ASS800) were examined with a scanning electron microscopy (JEOL JSM-5500 LV) coupled with energy-dispersive x-ray spectrometry.
Application in the real wastewater sample
Wastewater sample, from the heavy metals analysis laboratory, was treated by ASS800 adsorbent at the optimum conditions ofCd, Pb, Cu and Mn adsorption.
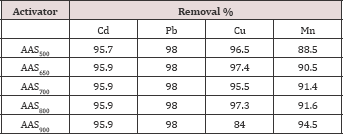
Table 2: Metal removal efficiency by the developed activated sewage sludge.
Results and discussion
Metal removal efficiency bythe developed adsorbents
Pyrolysis temperatures affected the surface area of the sewage sludge adsorbent. The adsorption data for the removal of Cd,Pb, Cu and Mn by the developed adsorbents (AAS900, AAS800, AAS700,AAS650, and AAS500), at the experimental optimum conditions are represented in Table 2. The data showed that all the ASS adsorbents, the adsorbent AAS800 have higher metals removal capability than with the other adsorbents, and so, this adsorbent AAS800 will be used for all experiments. The chemical composition of the prepared adsorpant (ASS800)is presented in Table 3.
Table 3: XRF data of chemical composition of adsorbent ASS(800).

Rozada et al. (2009) prepared sewage sludge adsorbents by physical activation with pyrolysis at 650 °C to remove Pb from aqueous solution, their results showed that physically activated sludge at 650 °C has an adsorption capacity of 30.1 mgg'1 which was lower than our result (46.7 mgg'1) by physically ASS at 650 °C. Hammaini et al. [13] used dried activated sewage sludge to remove Pb and Cd from aqueous solution, and found that the maximum Pb and Cd adsorption capacity were 143mgg'1 and 51.8mgg-1, respectively. These results were higher than that in our results by physically activated sewage sludges which ranged from 40.4 to47.8mgg-1 for Pb, and from 9.4 to 32.8mgg-1 for Cd. Bouzid et al. [14] prepared activated sewage sludge with physical activation at 600 °C to remove Cu, the estimated maximum adsorption capacity of Cu was 5.71mgg'1 which is lower than that in our result by physically ASS at 650 °C (20.2mgg'1). Seredych and Bandouz [15] prepared adsorbents from sewage sludge by the pyrolysis at 650 and 950 °C to remove Cu, the results showed that low pyrolysis temperature 650 °C results in adsorbent with Cu adsorption capacity (63.4mgg'1) , and this result was higher than that in our results using pyrolysis temperature 950 °C (34.0mgg'1).
Batch adsorption
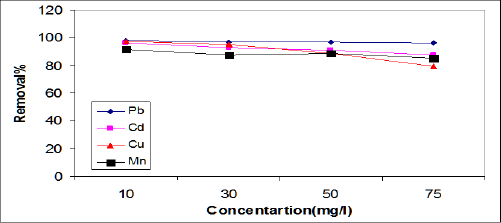
Effect of initial metal ion concentration: The adsorption data for the removal of Cd, Pb, Cu,and Mn by ASS800 adsorbent at the optimize experiment conditions is represented in Figure 1. The adsorption data show that with the increase of the initial concentration of Cd, Pb, Cu, and Mn from 10 to 75mgL-1, the percent removal of Cd, Pb, Cu, and Mn by ASS(800) decreased from 95.9 to 87.3%, 98 to 95.9%, 97.3 to 79%, and 91.6 to 84.8%, respectively. This means that the highest adsorption affinity occurred at lower metal concentration and this agreed with the results obtained by Faust and Aky [16]. This result may be explained by a limited availability of active sites on the ASS surface [17]. Xuejiang et al. [18] showed that the adsorption capacities of Cd and Cu on the surface of the dried activated sludge increased with the increase of initial metal ion concentration from 20 to 100 mg/L. The experimental data of [18-20] indicated that the adsorption capacity of Pb, Cd, and Cu on activated sewage sludge increases with the increase in initial metal concentration. Our results were agreed with all previous results.
Figure 1: Effect of initial metal ion concentration on removal efficiencies of ASS.
Effect of solution pH: The effect of pH on the adsorption efficiency of Cd, Pb, Cu, and Mn on ASS (800) adsorbent was studied in the pH range of 2-9 using 100mg/L of initial metal concentration, and represented in Figure 2. The removal of Cd, Pb and Cu increased with an increase of pH level up to pH 6, whileMn reached maximum adsorption at pH 8 as the result of Mn precipitation. The removal of Cd, Pb, Cu and Mn ions by sewage sludge adsorbent (ASS800) was explained by electrostatic attraction, surface complex formation and precipitation mechanism [21].
Figure 2: Effect of initial metal ion concentration on removal efficiencies of ASS.
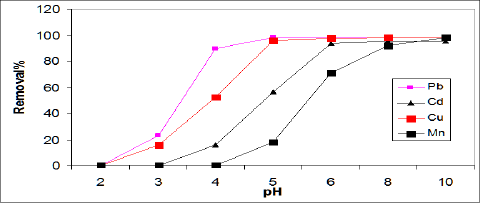
Majumdar et al. [22] reported the maximum sorption of Pb by biomass noted at pH 5-6. Otero et al. [8] showed that the maximum adsorption of Pb and Cu by activated sewage sludge was at pH 4. Rashed [17] showed that Pb adsorption on peach and apricot stones increased as pH increases from 3 to 6.5, and the maximum adsorption of Pb observed at pH 7. Bouzid et al. [14] showed that Cu adsorption using sewage sludge ash increased as pH increased and the maximum adsorption (99%) was at pH 7.2. Rozada et al. [8] showed that at pH values lower than 6.0 the removal of Cu by dry activated sludge was performed by biosorption mechanism, while above pH 6 metal precipitations occurs.
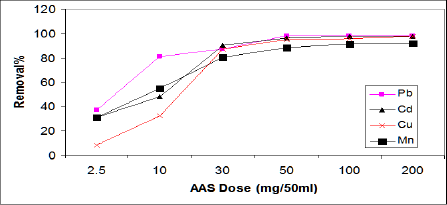
Effect of ASS800 dosage on metal ion adsorption: The effect of adsorbent amount on the adsorption of Cd, Pb, Cu, and Mn were studied for a variety of ASS800 adsorbent amount (g), and represented in Figure 3. The results show that the removal percent of Cd, Pb, Cu andMn increased with increaseASS800 adsorbent dose; this due to the availability of more surface functional groups and surface area at a higher adsorbent dose [23]. The optimum dosage of ASS800 for the removal of Cd, Pb, Cu, and Mn at experimental conditions is 50mg/50ml, with the removal percent of 95.5%, 98%, 97.6%, 91.5%, respectively. Zhai et al. [23] reported that increasing activated sewage sludge dosage increased Cd adsorption. Hammaini et al. [13] observed the variation of the sorption capacity for Pb, Cd and Cu versus activated sludge (biomass) dosage. Chenget al. [24] found that the residual concentration of Cu in synthetic wastewater decreased with the increase of sewage sludge ash, when the dosage reached 30-40g/L. Cu removal reached 99% at pH 4 and initial concentration 50mgl-1. Lian et al. [25] reported that the increasing of activated sewage sludge dosage from 1 to 6g has little effect on Curemoval, at the optimum dosage of 3g/100ml. Zehenze Li et al. [26] observed reduction in the adsorption amount of Mn from 99.5 to 10mg/g with increasing of the dosage of adsorbent (thermal decomposed leaf) from 0.6 to 5g/L, this result disagree with our result.
Figure 3: Effect of ASS dosage on the adsorption of Cd, Pb, Cu, and Mn.
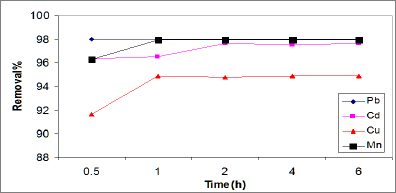
Effect of contact time on metal adsorption: The removal of Cd, Pb, Cu and Mn ions by ASS800 adsorbent as a function of contact time is presented in Figure 4. It was observed that the amounts of Cd, Pb, Cu and Mn ions adsorbed increased with an increase in contact time and gradually reached constant values within 1h. That result means that the adsorption equilibrium maintained at one hour. The fast adsorption at the initial stage may be due to the higher driving force making fast transfer of metal ions to the surface of adsorbent particles and the availability of the uncovered surface area and active sites on the adsorbent (Wu et al. [27]; Aroua et al. [28]). Lian et al. [25] observed little effect of contact time on Cu and Cd adsorption by activated sewage sludge. Cu adsorption reached up to 90% after 0.5h, while Cd adsorption (96% Cd) uptake is after contact time 7h. Rashed [17] reported that Pb adsorption on peach and apricot stones increased with time to reach its maximum that the equilibrium times for Mn and Cd by a clay mineral are 48 adsorption at 3 and 4h, respectively. Fonseca et al. [29] reported and 72h, respectively.
Figure 4: Effect of contact time on the adsorption of Cd, Pb, Cu, and Mn.
Effect of solution temperature on metal ion adsorption: The solution temperature has main effects on the adsorption process; in which it can affect the diffusion rate of the sorbate within the pores as a result of decreasing solution viscosity and also affect the number of the sorption sites generated because of breaking of some internal bonds near the edge of active surface sites of sorbent [30]. The adsorption data for the removal of Cd, Pb, Cu and Mn by ASS800 at a different solution temperature (25, 35, 45 and 55 °C) under the experimental conditions is represented in Figure 5.
Figure 5: Effect of solution temperature on the adsorption of Cd, Pb, Cu, and Mn.

The rate of metal removal increases with an increase in solution temperature from 25 to 35. The increase of metal adsorption with temperature could due to the strength of the binding and attraction between metal ions and active site on the surface of ASS800, where the decrease in metal adsorption with the rise of temperature may be due to the weakening of adaptive forces between the active sites of the adsorbents and adsorbate [31]. Lian et al. [25] observed that the biosorption of Cu by activated sewage sludge is maintained at about 94% when temperature changes from 10 to 40 °C. Ozdemir et al. [32] showed that no significant change in Mn, Cd, and Cu adsorption by dried powdered cell (microbial cells) when the temperature increase from 30 to 80 °C. Xuejiang et al. [18] reported that the sorption capacities of Cu and Pb by activated sewage sludge decreased with the increase of solution temperature which indicates an exothermic reaction.
Figure 6: SEM of raw and activated sewage sludge of Kima plant.
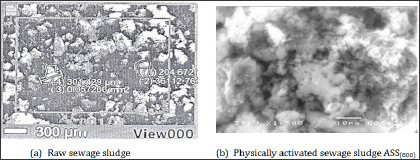
Morphological features: The micro-morphology and chemical composition of raw sewage sludge and ASS800 adsorbent were examined with ascanning electron microscopy coupled with energy-dispersive x-ray spectrometry. The results are represented in Figure 6 and Table 3. The scanning electron microscope (SEM) images as shown in Figure 6 revealed porous and irregular morphology of ASS800. Raw and activated sewage sludge seems as brown color particles. The data in Table 3 indicated that the main chemical composition of ASS800 is silica, iron and calcium in addition to a small percent of iron and aluminium.
Adsorption Isotherm
Langmuir, Freundlich and Scatchard isotherms were applied to describe the sorption equilibrium of metal ions on the adsorbent. The Freundlich expression is an empirical equation based on a heterogeneous surface, which relates that the concentration of a solute on the surface of an adsorbent, to the concentration of the solute in the liquid, Freundlich equation is given as follows:

Equation (1) can also be expressed in the linearized logarithmic form
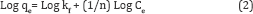
Where qe is the amount adsorbed (mg/g), Ce is the equilibrium concentration of the adsorbate (mg/l), and kf and n are the Freundlich constants related to adsorption capacity and adsorption intensity respectively, When Log qe is plotted versus Log Ce, the slop is equal to (1/n) and the intercept is equal to Log kf. The high value of R2 (Correlation coefficient of line) indicates that the adsorption follows Freundlich isotherm model perfectly [33]. When the value of (1/n) is between 0 to 1 indicate the heterogeneity of the sorbent, furthermore, the smaller 1/n and larger Kf values for sorbent indicate that sorbent has higher adsorption capacity, intensity and affinity for metal ion than the other types of sorbents .
Langmuir equation expressed as follows
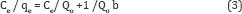
Where Ce (mg/l) is equilibrium concentration, qe (mg/g) the amount adsorbed at equilibrium, b (L/mg) is the Langmuir constant which related to the affinity of the binding site, and Qo (mg/g) is the maximum adsorption capacity parameter. When Ce/ qe is plotted versus Ce, the slope is equal to (1/Qo) and the intercept is equal to 1/Qo b.
The Scatchard plot analysis is a widely used technique in evaluating the main interaction types taking a role in a particular sorption process and dependence of binding types to experimental conditions, the Scatchard isotherm represents intermediate situations close to the Langmuir model. The shape of Scatchard plot is related to the type of the interactions of analyte with adsorbent. The presence of a deviation from linearity on a plot based on Scatchard analysis usually points out the presence of more than one type of the binding sites. Whilst the linearity of the Scatchard plot indicates that the binding sites are identical and independent. So, if the Scatchard plot is liner with a negative slop, it is related to interaction between the analyte and the binding sites follows the Langmuir model. When the Scatchard plot exhibits a deviation from linearity the interaction follows the analysis of data in terms of the Freundlich model [34].
The Scatchard isotherm equation for a linear plot [35,36] is represented by the following equation:

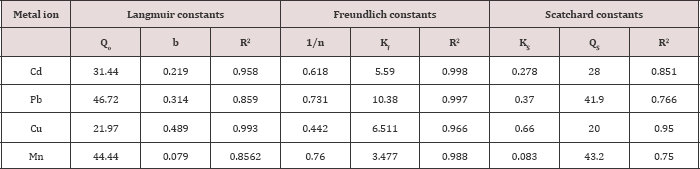
Where QS and Ks are Scatchard constants, related to the maximum monolayer adsorption capacity and equilibrium adsorption constant respectively. The results obtained on the adsorption of Cd, Pb, Cu, and Mn by ASS800 at different initial metal ion concentrations( 10, 30, 50 and 75mgL-1) was analyzed by models given by Freundlich, Langmuir and Scatchard , and represented in Table 4.
From Freundlich constants, the values of 1/n for adsorption on ASS800 were 0.618 for Cd, 0.731 for Pb, 0.442 for Cu and 0.760 for Mn. The maximum adsorption from Langmuir parameters for Cd, Pb, Cu, and Mn on ASS (800) are 31.4, 46.7, 21.9, and 44.4mg/g. These values indicated that the order of metal ions according to their affinity to adsorption on ASS800 is Pb>Mn>Cd>Cu. From Scatchard parameters the values of R2 for adsorption on ASS800 were 0.851 for Cd, 0.766 for Pb, 0.950 for Cu and 0.750 for Mn (Table 4) [37,38].
Table 4: Langmuir, Freundlich and Scatchard isotherm constants for adsorption of Cd, Pb, Cu, and Mn by ASS(800).
Application of real wastewater sample
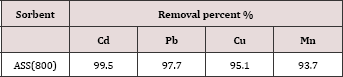
The possibility of the utilization of ASS800 as an effective adsorbent for the removal of Cd, Pb, Cu, and Mn was studiedby the application on real wastewater sample (Lab wastewater) containing these metals. The concentrations of metal ions in the Lab wastewater sample before and after the treatment with ASS800 are represented in Tables 5 & 6. The results revealed the possibility of the utilizationASS800 as an effective adsorbent for the removal of Cd, Pb, Cu, and Mn from polluted water.
Table 5: Physical and chemical analysis of lab. wastewater before treatment with activated sewage sludge.

Table 6: The removal percent of metal ions (Cd, Pb, Cu, and Mn) after treatment of lab. Waste water with ASS(800).
Conclusion
The present study showed the possibility of utilizing the activated sewage sludgeASS800, which derived from sewage sludge waste, as an active adsorbent for the removal of Cd, Pb, Cu, and Mn from polluted water. The results of this study indicated that the developed adsorbent, from activated sewage sludge, showed good ability to remove Cd, Pb, Cu, and Mn at low and high concentrations. The initial metal concentration, pH value, contact time and ASS(800) dosage exhibited significant effect on the adsorption process of Cd, Pb, Cu and Mn by ASS800. The adsorption data of Cd, Pb, Cu, and Mn on ASS800 were fitted well the Freundlich model and Langmuir equations.
Read More About Lupine Publishers Journal of Organic and Inorganic Chemical Sciences Click on Below Link:
https://lupinepublishers-chemicalsciences.blogspot.com/

No comments:
Post a Comment
Note: only a member of this blog may post a comment.