Lupine Publishers | Journal of Food and Nutrition
Abstract
This is out line work on the process development for the synthesis of buprenorphine, naltrexone, naloxone, and nalbuphine from naturally occurring opiates such as Thebaine, also known as codeine methyl enol ether, and oripavine. Several new methods for N-demethylation of morphinans have been developed during the pursuit of this research. The article traces the evolution of various approaches and provides a comparison for overall efficiency. A reverse phase high performance liquid chromatographic (RPHPLC) method has been developed and validated for simultaneous estimation of Naloxone Hydrochloride and Buprenorphine Hydrochloride in pure and marketed formulations. Separation was carried out using column Hypersil ODS C18 (250 mm x 4.6mm x 5μm particle size) in isocratic mode using mobile phase composition pH 6.0 ammonium acetate Buffer: Acetonitrile (68:32)v/v and UV detection at 310nm. The compounds were eluted at a flow rate of 1.0mL/ min. The average retention times for Naloxone and Buprenorphine were 2.86 and 3.67 min, respectively. The method was validated according to the ICH guidelines. The percentage RSD of all validation parameters found to be less than 2% indicating high degree of accuracy and precision of the proposed HPLC method. The method was linear over the concentration of 5.30μg/ml and 20-120μg/ml for Naloxone and Buprenorphine respectively. The LOD and LOQ of Naloxone were found to be 0.08μg/mL and 0.26μg/mL and of Buprenorphine were found to be 0.0078μg/mL and 0.0237μg/mL. The drugs were also exposed to acidic, alkaline, oxidative, thermal and photolytic conditions and the stressed samples were analyzed by the proposed method. Degradation studies showed that the both the drugs were highly stable under acidic, oxidative, thermal and photolytic conditions. Under alkaline conditions, RT values were shifted to lower as compared to standard without any additional peaks. The high percentage of stability under stress conditions confirms the suitability of the method for simultaneous estimation of Naloxone Hydrochloride and Buprenorphine Hydrochloride in pure and marketed formulations.
Keywords: Analgesics, Opiate-derived pharmaceuticals, Morphine alkaloids, Buprenorphine hydrochloride, Naloxone hydrochloride, RP-HPLC, Validation and degradation studies, ICH Guidelines semisynthesis, Process development
Abbrevations: LOD: Limit of Detection, LOQ: Limit of Quantification, RPHPLC: Reverse Phase High Performance Liquid Chromatographic Method
Introduction
Buprenorphine, Naltrexone, Naloxone, and Nalbuphine have been used for centuries for their analgesic effects and are considered to be the most commonly used pharmacologic agents for the management and treatment of moderate to severe pain. Although these agents are considered safe when used properly and under physician supervision, it is important to understand the risks and benefits associated with prescribing the agents in this pharmacologic class.
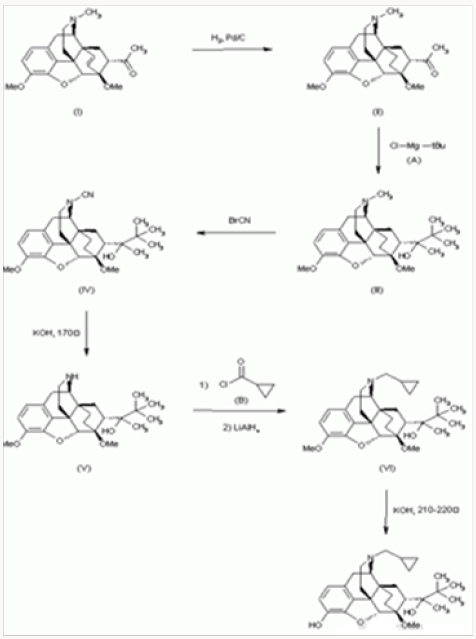
Buprenorphine Synthesis Method:
The hydrogenation of 7-acetyl-6,14-endoetheno-6,7,8,14- tetrahydrothebaine
a) With H2 over Pd/C in acetic acid gives the corresponding endo-ethano derivative
b) Which By a Grignard reaction with tertbutyl-magnesium chloride
i. In ether-benzene yields 7alpha-(2-hydroxy-3,3-dimethyl- 2-butyl)-6,14-endo-ethano-6,7,8,14 -tetrahydrothebaine
c) The reaction of III with BrCN in CH2Cl2 affords 7alpha- (2-hydroxy-3,3-dimethyl-2-butyl)-6,14-endo-ethano-Ncyano- 6,7,8 ,14-tetrahydronorthebaine
d) Which is treated with KOH in ethylene glycol at 170 C to give 7alpha(2-hydroxy-3,3-dimethyl-2-butyl)-6,14-endoethano- 6,7, 8,14-tetrahydronorthebaine
e) This compound is treated first with cyclopropylcarbonyl chloride
ii. In CH2Cl2 containing triethylamine, followed by a reduction with LiAlH4 in refluxing THF yielding N-cyclopropylmethyl- 7alpha-(2-hydroxy-3,3- Dimethyl-2-butyl)-6,14-endo-ethano- 6,7,8,14-tetrahydro-northebaine
f) Finally,
g) Is demethylated with KOH in diethyLene glycol at 210- 220 0C.
Drugs Fut 1977, 2, (9): 570
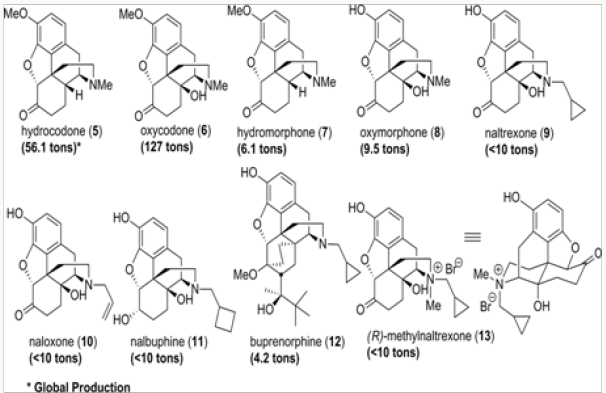
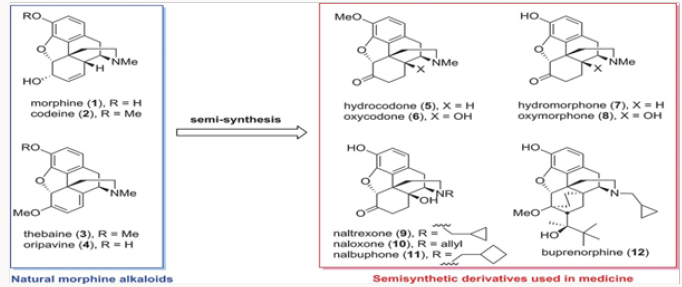
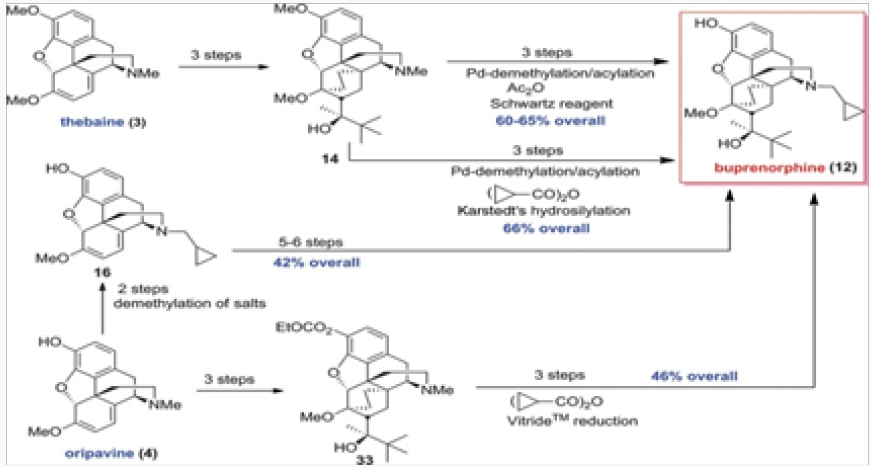
All medicinal opiate agents in use today are derived from naturally occurring morphine alkaloids by semisynthesis, as shown in an abbreviated fashion in (Figure 1) [1]. Two important transformations are required: the first is the oxidation of the C-14 position to C-14 hydroxyl and the second is the replacement of the N-methyl functionality with other alkyl groups. The former process has been reduced to practice in several efficient ways, while the latter suffers from the use of toxic reagents and multistep operations [2]. The global production figures for various analgesics and antagonists derived from natural morphinans are shown in (Figure 2a) Clearly the scale of semisynthesis requires that efficient and environmen- tally benign manufacturing protocols be developed. Crucial to all procedures are the methods employed for N- and O-demethylation of the alkaloids or any advanced synthetic intermediates. In this review, we trace several generations of improvements in the preparation of buprenorphine, naltrexone, methyl naltrexone, naloxone, and nalbuphine. In all of these projects, new demeth- ylation protocols have been developed and major improvements in efficiency have been attained (Figure 2b).
The First Generation
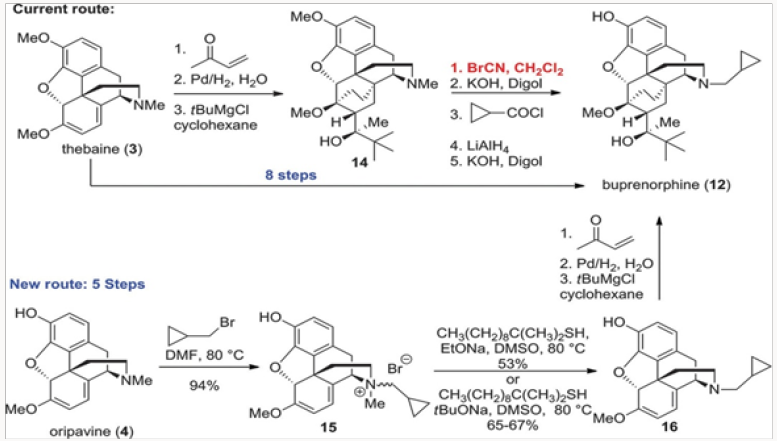
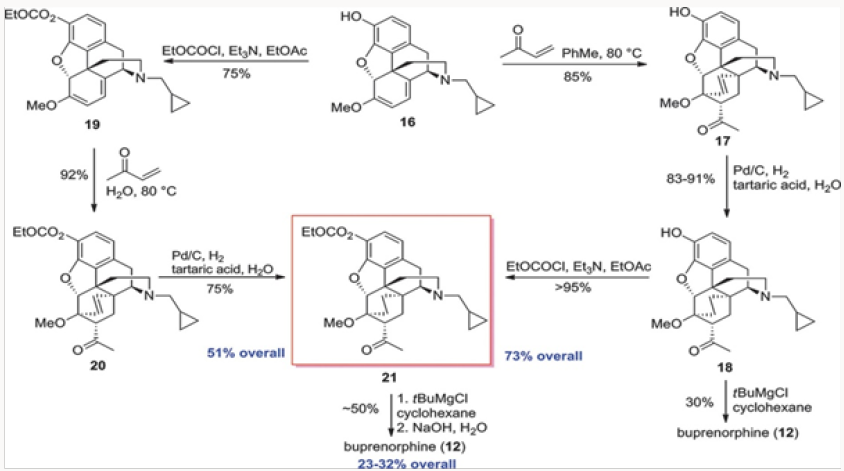
The first project undertaken was aimed at improvement of the commercial synthesis of buprenorphine. This route takes eight steps form Thebaine, also known as codeine methyl enol ether, as shown in (Scheme 1.2) It should be noted that five of the eight steps are required for N- and O-demethylation reactions. The former process involves the von Braun reaction [3] with cyanogen bromide, which poses toxicity issues. We have shortened the synthesis by starting with oripavine and performing selective N-demethylation of the diastereomeric salts 15 generated by alkylation of oripavine with cyclopropylmethyl bromide. The N-demethylation with sodium thiolates (produced from the thiol with either MeONa or tBuONa) proceeded in agreement with previously published protocols [4] to afford cleanly N-cyclopropylmethyl nororipavine 16 in yields of 53%-67% [5]. We noted that the two diasteroemers of 15 reacted at different rates.
The conversion of 16 to buprenorphine was carried out in three steps in analogy to those employed in the commercial route from Thebaine, also known as codeine methyl enol ether, namely Diels- Alder cycloaddition, hydrogenation, and Grignard addition. Three routes were examined for the conversion of 16 to buprenorphine. The first was the conversion of 16 to ketone 18 and direct treatment of 18, without the protection of the phenol, with the Grignard reagent to produce buprenorphine in ~30% yield (Scheme 2). In the other two routes the phenol was protected as a carbonate at the stage of either 18 or 19. The two routes intersected at 21, which was converted to buprenorphine in two steps, with the route through 18 providing a better overall yield [6,7].
Literature survey reveals that there were number of analytical methods available for both the drugs alone or in combination with other drugs including spectroscopy, chromatographic methods such as gas chromatography with electron-capture or mass spectrometry detection and HPLC with fluorescence electrochemical or mass spectrometry detection [8-13] are reported, but there is no method established for the stability indicating RP-HPLC under stress for this combination. The present work describes the development of stability indicating RP-HPLC method, which can quantify these components simultaneously from a combined dosage form. The present RP-HPLC method was validated [14-15] and applied under stressed conditions according to (ICH) guidelines. ICH has made the mandatory need of developing stability indicating assay methods for every drug candidates. Stability indicating assay methods helps in establishing the inherent stability of the drug which provides assurance on detection changes in identity, purity and potency of the product on exposure to various conditions [16]. So an attempt has been made to develop a method under stress conditions like acidic, basic, thermal, photolytic and oxidative, this which in turn can help in establishing the degradation pathways and the intrinsic stability of the molecules. The object of the present work was to develop a stability indicating method for the simultaneous estimation of Naloxone hydrochloride and Buprenorphine hydrochloride.
Materials and Methods
Experimental Instruments and Columns
Shimadzu with high pressure liquid chromatographic instrument provided with a LC 20 AD Pump and Prominence SPD 20A UV-deuterium detector. Data acquisition was performed by using Spin chrome software, Shimadzu Class VP version 6.12 SPS data system. Power Sonicator, model no: 405, Hwashin Technology, Korea. The column used in the development for determination is Hypersil ODS C18 (250mm x 4.6mm; 5μm) [17].
Chemicals Used
HPLC grade Acetonitrile, methanol and water were purchased from E.Merck Co; Mumbai, India and Ammonium acetate, glacial acetic acid AR grade were purchased from SD Fine Chem. Mumbai, India. The reference samples of Buprenorphine Hydrochloride and Naloxone Hydrochloride were supplied by Spectrum Analytical Labs, Hyderabad, Telangana State, India, and branded formulation was purchased from local market [18].
Chromatographic Conditions
An ideal wavelength is one that uses good response for the drugs to be detected Buprenorphine Hydrochloride and Naloxone Hydrochloride in diluents the spectra was scanned on UV-visible spectrophotometer in the range of 200nm to 400nm against diluents as blank. The maximum absorbance of both drugs was found to be at 310nm. So the 310nm was selected for simultaneous estimation Buprenorphine Hydrochloride and Naloxone Hydrochloride in Pharmaceutical Dosage Forms (Table 1).
Preparation of Mobile Phase
Weighed accurately 770mg of ammonium acetate and dissolved in 100ml of water and volume was made up to 1000mL with water adjust the PH to 6.0 using glacial acetic acid. The solution was filtered through 0.45μ membrane filter and was degassed [19]. A freshly prepared binary mixture of Ammonium acetate and glacial acetic acid buffer: Acetonitrile in a ratio of (68:32) V/V was used as the mobile phase. Methanol was used as diluents for preparing the working solution of the drug. The mobile phase was filtered through 0.05μ membrane filter and sonicated. The flow rate of the mobile phase was maintained at 1.0mL/min. The column temperature was maintained at 30ºC and the detection of the drug was carried out at 310 nm.
Preparation of stock solution
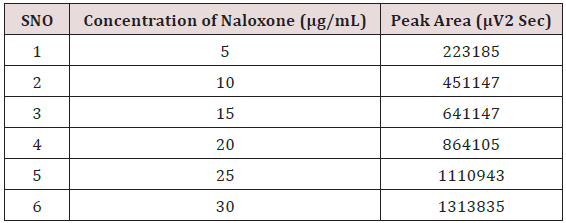
Weighed accurately about 5mg of Naloxone Hydrochloride and 20mg Buprenorphine Hydrochloride transferred into 25mL volumetric flask. The solution was sonicated and filtered through Whatman filter paper; resulting solution was diluted with the mobile phase to get a working standard solution.1mL from the above Stock solutions of Naloxone Hydrochloride and Buprenorphine Hydrochloride was taken into a 10mL volumetric flask and diluting up to the mark with the mobile phase. Mixed standard solutions of different concentrations ranging from 5-30μg/mL of Naloxone Hydrochloride and 20-120μg/mL of Buprenorphine Hydrochloride were prepared by taking suitable aliquots of working standard solution in different 10mL volumetric flasks and diluting up to the mark with the mobile phase [20-25].
Preparation of sample solution
Twenty tablets were weighed and average weight was determined and finally powdered. Tablet powder equivalent to 0.5mg Naloxone Hydrochloride and 2mg Buprenorphine Hydrochloride was accurately weighed and transfer to 10mL volumetric flask. The contents were sonicated for about 15min for complete solubility of the drug after adding 10mL of mobile phase and the volume was made up to the mark with mobile phase. Then the mixture was filtered through a 0.45μ membrane filter. From the above solution, 4mL aliquot was taken into a separate 10 mL volumetric flask and diluted up to the volume with the mobile phase and mixed well.
Optimization of HPLC method
The HPLC method was optimized with an aim to develop an accurate and precise method for the estimation of Naloxone Hydrochloride and Buprenorphine Hydrochloride in pharmaceutical dosage forms. For the method optimization different mobile phases were tried but acceptable retention times, theoretical plates and good resolution observed with pH 6.0 ammonium acetate buffer: Acetonitrile in a ratio of (68:32) v/v was used as the mobile phase using Hypersil ODS C18, 250 X 4.6mm, 5μm (Tables 2 & 3).
Linearity
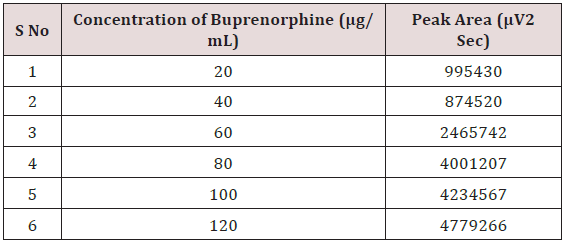
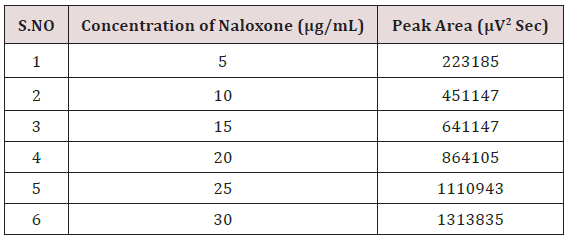
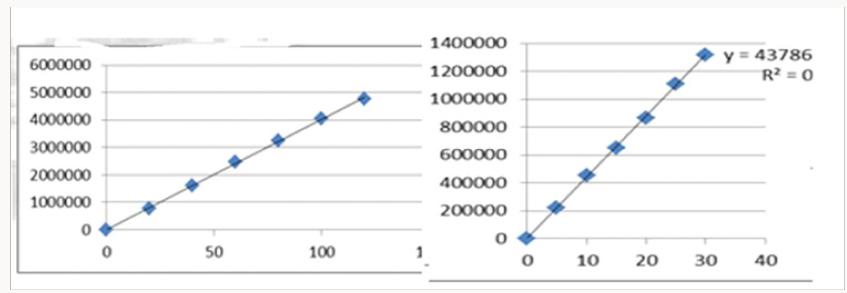
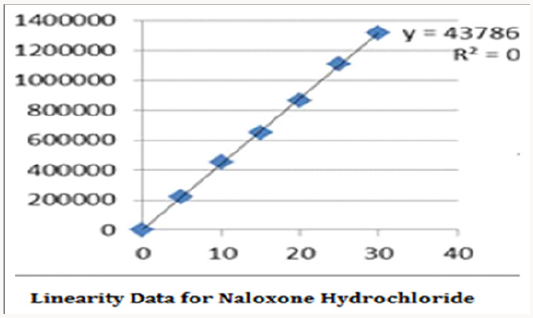
Alinear relationship was evaluated across the range of the analytical procedure with a minimum of six concentrations. A series of standard dilutions of NAH and BUH were prepared over a concentration range of 5-30μg/mL and 20-120μg/mL from stock solution and injected. Linearity is evaluated by a plot of peak areas as a function of analyte concentration, and the results were evaluated by using the statistical methods like slope, intercept, and regression (R2) correlation coefficients (R) and the data was given in (Tables 2-4) (Figures 3 & 4). Repeatability expresses the precision under the same operating conditions over a short Interval of time. The six repeated homogenous injections of standard solutions were made about 20μg/mL Naloxone and Buprenorphine 80μg/mL and the response factor of drug peaks, mean, standard deviation and percentage RSD were calculated. Repeatability data for NAH and BHU are summarized in (Table 4).
Method Precision
Method precision was determined by injecting six sample solutions of Single batch were analysed as per test method. The mean, standard deviation and percentage RSD for peak areas of Naloxone and Buprenorphine from sample solutions were calculated. The results were given in the Table 4 [26,27].
Accuracy
Accuracy determination, three different concentrations were prepared separately i.e.50%, 100%, and 150% of analyte and the chromatograms were recorded for the same. The results obtained for recovery were found to be within the limits. The results were given in the Table 5 [28,29].
Robustness
To evaluate the robustness, the following small deliberate variations are made in the method and analyzed the sample in triplicate. 1. Column oven temperature (±50C), 2.Flow rate (±10%), 3.change in buffer composition (±5%). The system suitability was evaluated in each condition and compared with the results of method precision. The results were given in the Table 6 [30,31].
Specificity
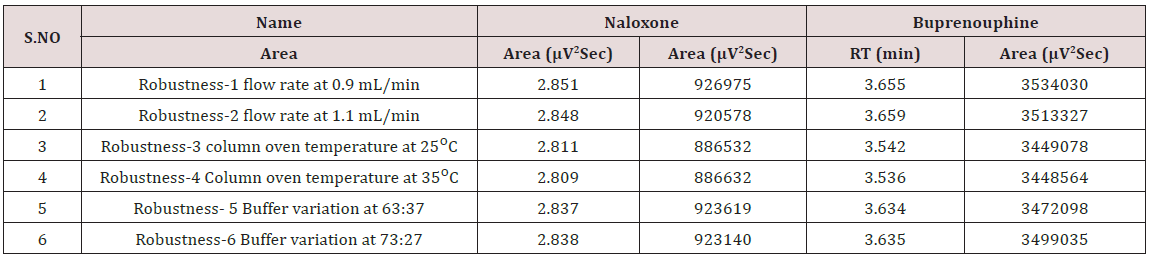
Specificity shall be established by demonstrating that the procedure is unaffected by the presence of interference at the retention time of the Naloxone Hydrochloride and Buprenorphine Hydrochloride with respect to mobile phase, Diluents, placebo and degradants. The specificity studies include deliberate degradation of the tablet sample by exposure to stress conditions, Specificity studies also include blank, placebo solution, and sample solution (control sample), Naloxone and Buprenorphine standard solution were injected into the HPLC system. There was no interference from the blank and placebo at the retention time of the peaks. Peak purity data reveals that Naloxone and Buprenorphine were homogeneous and there was no interference at the retention time of both drug peaks (Figure 5).
Degradation Studies
Forced degradation or stress studies are undertaken to demonstrate specificity. The objective of developing stabilityindicating methods was particularly little information is available about potential degradation products. These studies also provide information about the degradation pathways and degradation products that could form during storage. Forced degradation studies may help facilitate pharmaceutical development as well in areas such as formulation development manufacturing and packaging in which knowledge of chemical behavior can be used improve a drug product [32-34].
Forced Degradation study was carried out by treating the sample under the following conditions [16]. Twenty tablets were weighed and average weight was determined and finally powdered. Tablet powder equivalent to 0.5mg Naloxone Hydrochloride and 2mg Buprenorphine Hydrochloride was accurately weighed and transfer to 10mL volumetric flask. The contents were sonicated for about 15 min for complete solubility of the drug after adding 10 mL of mobile phase and the volume was made up to the mark with mobile phase. Then the mixture was filtered through a 0.45μ membrane filter. From the above solution, 4mL aliquot was taken into a separate 10 mL volumetric flask and diluted up to the volume with the mobile phase and mixed well.
Acid Degradation
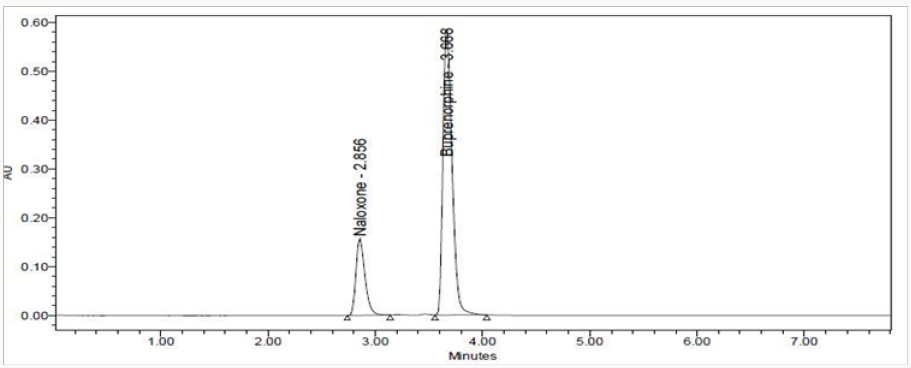
To 1ml of stock solution of NAH and BUH, 1ml of 2N Hydrochloric acid was added and refluxed for 2hrs at 600C.The resultant solution was diluted to obtain 20μg/ml & 80μg/ml solution and10μl solutions were injected into the system and the chromatograms were recorded to assess the stability of sample (Figure 6) [35]
Alkali Degradation
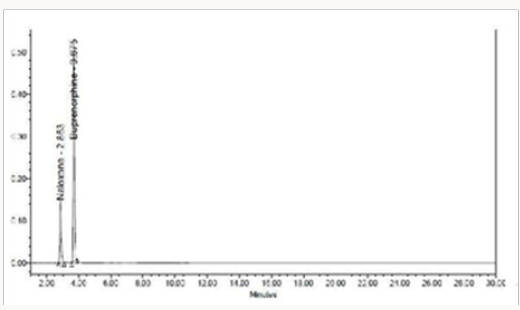
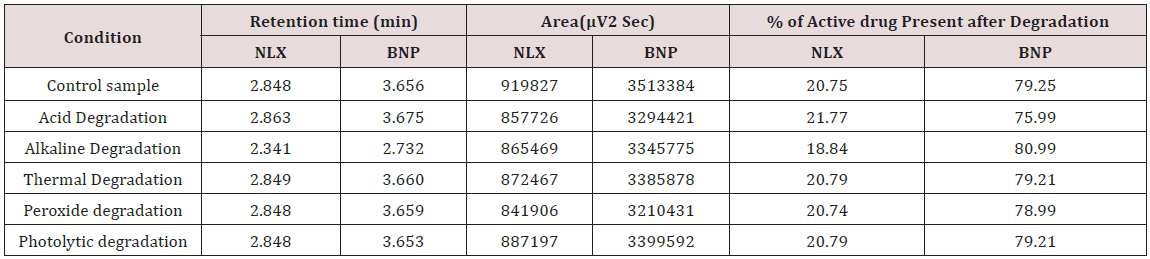
To 1ml of stock solution Naloxone and Buprenorphine, 1ml of 2N sodium hydroxide was added and refluxed for 2hrs at 600c. The resultant solution was diluted to obtain 20μg/ml & 80μg/ml solution and 10μl were injected into the system and the chromatograms were recorded to assess the stability of sample Figure 7 [36].
Thermal Degradation
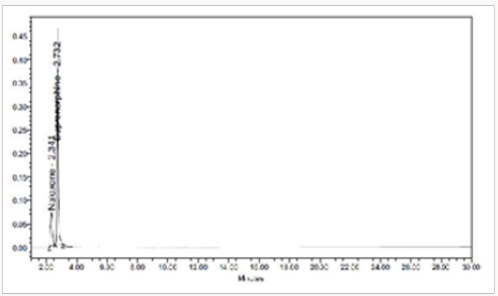
The standard drug solution was placed in oven at 105 °C for 6 hrs to study thermal degradation. For HPLC study, the resultant solution was diluted to 20μg/ml & 80μg/ml solution and 10μl were injected into the system and the chromatograms were recorded to assess the stability of the sample Figure 8 [37].
Peroxide Degradation
To 1ml of stock solution of Naloxone and Buprenorphine, 1 ml of 20% hydrogen peroxide (H2O2) was added separately. The Solutions were kept for 1hr at 600c. For HPLC study, the resultant solution was diluted to obtain 20μg/ml & 80μg/ml solution and 10μl were injected into the system and the chromatograms were recorded to assess the stability of sample Figure 9 (Table 7).
Photo Stability
The photochemical stability of the drug was also studied by exposing the 20μg/ml & 80μg/ml solution to UV Light by keeping the beaker in UV Chamber for 7 days or 200 Watt hours/m2 in photo stability chamber. For HPLC study, the resultant solution was diluted and 10μl were injected into the system under stabilized chromatographic conditions Figure 10.
Limit of Detection (LOD) and Limit of Quantification (LOQ)
The LOD and LOQ of the developed method were determined by analysing progressively low concentration of the standard solutions using the developed methods. The results are given in the Table 4 LOD= 3.3σ/S and LOQ=10σ/Sσ = standard deviation of the response S = slope of the calibration curve of the analyte.
Analysis of Marketed Formulations
The fixed chromatographic conditions were applied for the estimation of Naloxone and Buprenorphine (0.5mg of Naloxone and 2mg of Buprenorphine) formulation by RP-HPLC method. Twenty tablets were weighed and average weight was determined and finally powdered. Tablet powder equivalent to 0.5mg Naloxone Hydrochloride and 2mg Buprenorphine Hydrochloride was accurately weighed and transfer to 10mL volumetric flask. The contents were sonicated for about 15min for complete solubility of the drug after adding 10mL of mobile phase and the volume was made up to the mark with mobile phase. Then the mixture was filtered through a 0.45μ membrane filter. From the above solution 4mL aliquot was taken into a separate 10mL volumetric flask and diluted up to the volume with the mobile phase and mixed well. Initially, inject 20μL of blank solution, placebo solution, sample solution and standard solution, Disregard peaks due to blank and placebo if any. The results were given in the table 8.
Recording of chromatograms
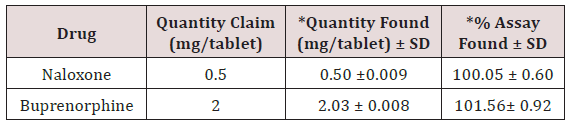
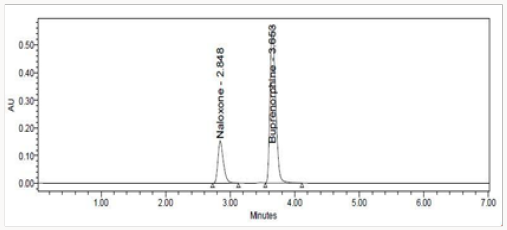
The standard solutions stabilize the system until stable obtained. Initially, inject the blank solution and placebo. The standard chromatograms were recorded by injected standard solutions and the peak areas of standard chromatograms were noted. Calibration graph was plotted using peak area versus concentration. Then the sample solution was injected and the amount of Naloxone and Buprenorphine present in the formulation was calculated from the calibration curve. The amount of Naloxone and Buprenorphine present in per tablet Naloxone and Buprenorphine was found to be 0.50 ± 0.009mg and 2.03 ± 0.008mg. Total label claim for (0.5mg of Naloxone and 2mg Buprenorphine) formulation Figure 11.
Result and Discussion
The goal of the study is to development of simple, rapid, sensitive, specific and accurate HPLC methods for the routine quantitative determination of samples. Hypersil C18 ODS Column (250 mm x 4.6 mm; 5μm) as stationary phase. The mobile phase composition ammonium acetate and glacial acetic acid buffer: Acetonitrile in the ratio of 68:32 and pH adjusted to 6.0±0.1 with glacial acetic acid selected. A good linear relationship (r2 = 0.9995 & r2 = 0.9996) was observed in the range of 5-30μg/mL & 20μg/ mL-120μg/mL for NAH and BUH (Figures 3 & 4) Linear Recovery values obtained by the proposed method is accurate. The system precision was established by six replicate injections of the standard solutions containing analyte of interest. The value of relative standard deviation of Naloxone and Buprenorphine was found to be 0.72 and 0.7 within the limit, indicating the injection repeatability of the method. The method precision was established by carrying out the analysis six times using the proposed method. The relative standard deviation of Naloxone and Buprenorphine was found to be 0.6 and 0.9 within the limit, indicating the injection repeatability of the method (Table 4). Six samples of the same batch were prepared on different days by the analysts. Calculated percentage RSD for two different days in six samples for ruggedness results with the method precision within the limits. The system suitability was evaluated in each condition and compared the results with method precision results. The method is robust for change in wavelength, mobile phase composition and column oven temperature. The specificity studies include deliberate degradation of the tablet sample by exposure to stress conditions, Specificity studies also include blank, placebo solution and sample solution (control sample), NAH and BUH standard solution were injected into the HPLC system. There was no interference from the blank and placebo at the retention time of the peaks. Peak purity data reveals that Naloxone and Buprenorphine were homogeneous and there was no interference at the retention time of both drug peaks. The method does not permit detection of any degradation products for NAH and BUH after subjecting to various degradation procedures like acid, base, thermal, peroxide and photolytic degradations, the stressed samples were analyzed by the proposed method. Degradation studies showed that the both the drugs were highly stable under acidic, oxidative, thermal and photolytic conditions without any change in RT values but under alkaline conditions RT values were shifted to lower as compared to standard without any additional peaks (Figures 6-10).
Figure 13: Opiate-derived pharmaceutical agents. Numbers in parentheses represent the global production figures.
The high percentage of stability under stress conditions confirms the suitability of the method for simultaneous estimation of Naloxone Hydrochloride and Buprenorphine Hydrochloride in pure and marketed formulations. The formulation was calculated from the calibration curve. The amount of Naloxone Hydrochloride and Buprenorphine Hydrochloride in per tablet Naloxone and Buprenorphine was found to be 0.50±0.009 mg and 2.03±0.008 mg. Total label claim for 0.5mg Naloxone and 2mg Buprenorphine of formulation (Figures 12-14).
The Second Generation
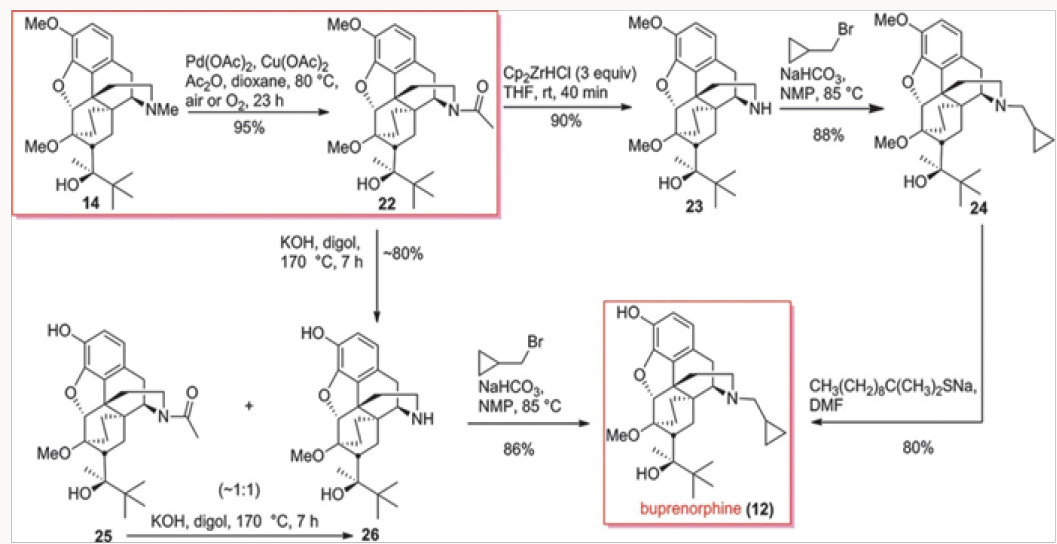
Another approach to buprenorphine involved the palladiumcatalyzed N-demethylation/acylation of the advanced commercial intermediate 14, as shown in Scheme 3. This process was discovered in 2008 in conjunction with the palladium-catalyzed N-demethylation/acylation of hydrocodone. Treatment of amide 22 with Schwartz reagent provided the secondary amine 23, whose alkylation and O-demethylation led to buprenorphine in good yields.7 The O-demethylation also occurred under basic con- dition during the hydrolysis of the acetamide in 22. Both routes depicted gave buprenorphine in good overall yields.
The Third Generation
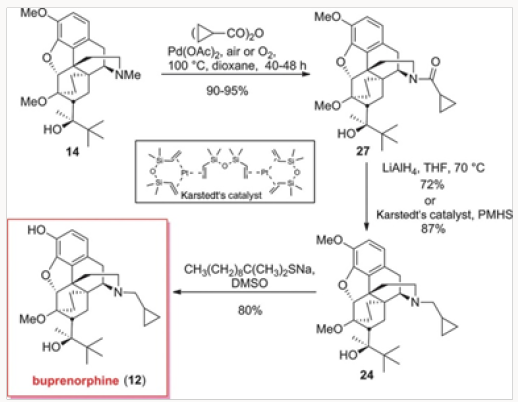
The palladium-catalyzed process was repeated with the anhydride derived form cyclopropylcarboxylic acid, as shown in (Scheme 4). The advanced inetermediate 14 provided the cylopropylcarboxamide 27 in excellent yield. Reduction either with LiAlH4 or by hydrosilylation gave 24, which was converted to buprenorphine by O-demethylation [7] (Figures 15-16).
The Fourth Generation
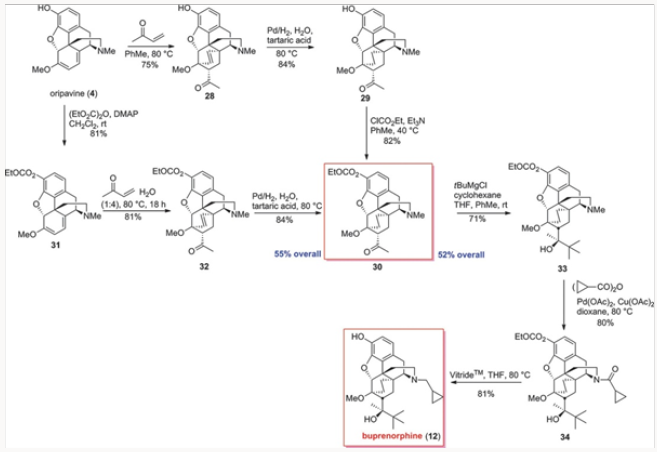
The palladium-catalyzed N-demethylation/acylation leading to cylopropyl carboxamide 27 was next applied to the carbonateprotected derivative 33 derived from oripavine. We wished to compare the overall efficiency of approaches to buprenorphine from both Thebaine, also known as codeine methyl enol ether, and oripavine, as the latter approach would not re- quire O-demethylation. Thus, ketone 30 was synthesized by two routes proceeding in a comparable yield, as shown in Scheme 5, and converted to 33 by the action of the Grignard reagent. The N-demeth- ylation/acylation produced in high yield 34, whose reduction and concomitant removal of the carbonate gave buprenorphine.7Comparison of approaches to buprenorphine
The four generations of approaches to buprenorphine have been evaluated for overall efficiency, as shown in Fig. 3. Although all routes are six steps in length, it would appear that the best overall yield is provided in the palladium-catalyzed N-demethylation/ acylation of the advanced intermediate 14. The issue of actual cost of each of these routes has not been addressed in detail but it is clear that reagent and catalyst costs will be major considerations for eventual scale-up and production. Without the evaluation of cost parameters the overall yield figures are not the most important factor. Suffice it to say that all four approaches provided significant improvements over the commercial route.
Naltrexone8
We next addressed the synthesis of naltrexone and eventually also (R)-methyl naltrexone. Several different approaches were investigated and compared for overall merit.
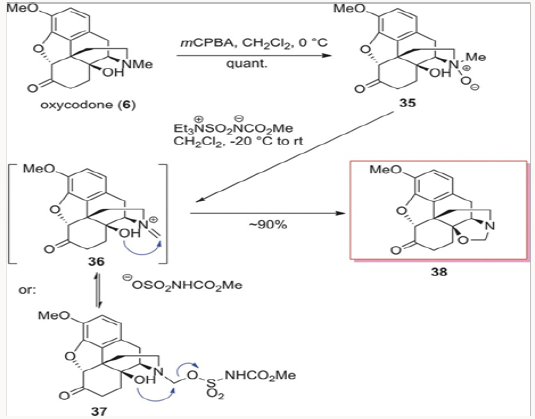
N-demethylation of N-oxides with the Burgess reagent
This rather interesting demethylation method is shown in Scheme 6, applied to the N-oxide derived from oxycodone 6. Treatment of N-oxide 35 with the Burgess reagent provided cleanly oxazolidine 38, presumably via the closure of the C-14 hydroxyl on the imminium species 36 or the intermediate trapping of such species with the sulfonate followed by intramolecular displacement, as shown in Scheme 6.9 The generation of oxazolidine 38 was very exciting, as it led to several different methods of synthesis for naltrexone, naloxone, and nalbuphine. The most important benefit of the Burgess demethylation protocol was the fact that the carbon of the original N-methyl group remained in oxazolidine 38 after the demethylation and was not lost from the overall mass of the molecule as it had been during the previously applied N-demethylation methods. This simple fact would have a major impact on a general method of synthesis for the opiate-derived agents and will be discussed further in this review.
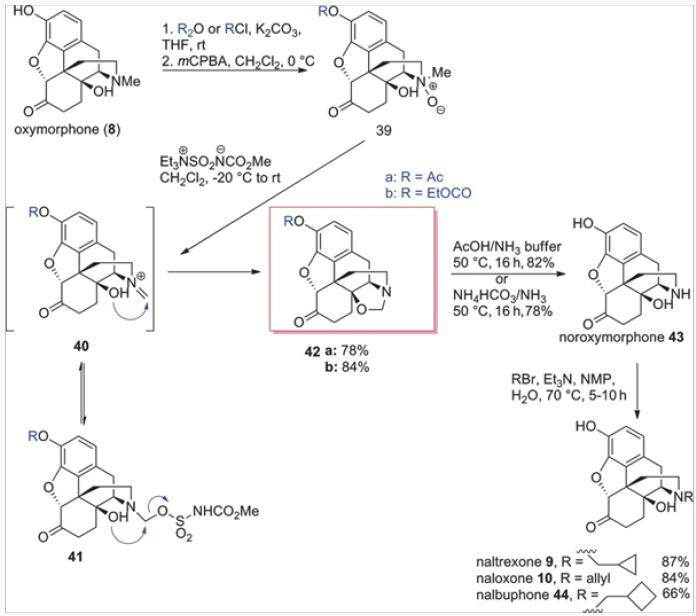
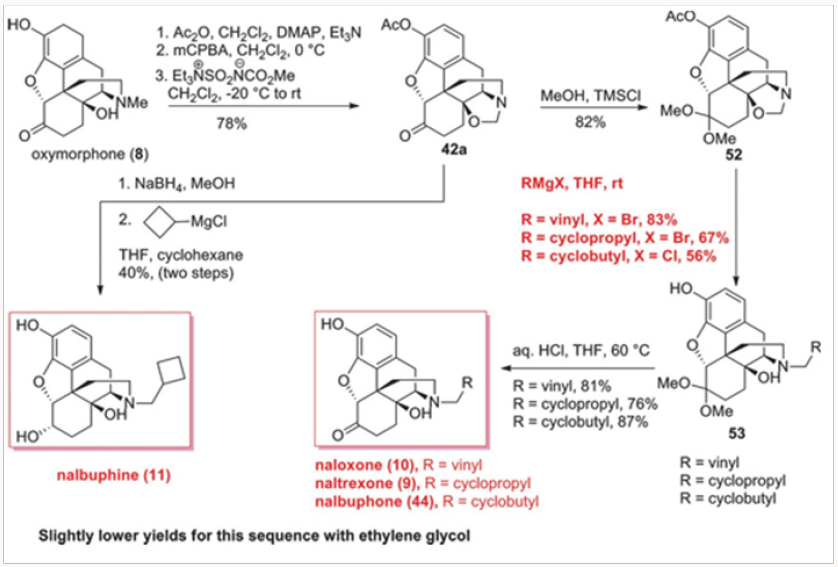
Similarly, the Burgess demethylation process was applied successfully to oxymorphone to produce oxazolidine 42a or 42b in which the phenolic function was protected either as an acetate oras a carbonate. Either of the oxazolidines 42 was easily converted to noroxymorphone 43, in which the secondary amine was avail- able for alkylation to either naltrexone 9, naloxone 10, or nalbu- phone 44, as shown in Scheme 7. Of special note is the fact that naloxone could not be obtained by N-demethylation of diastereomeric salts of type 15 because the rate of the corresponding deallylation would always be faster. The oxazolidines of type 42 proved to be very useful in another general approach to several opiate-derived medicinal agents, as will be shown further in this review (Figures 17-21).
N-demethylation/acylation approach to naltrexone
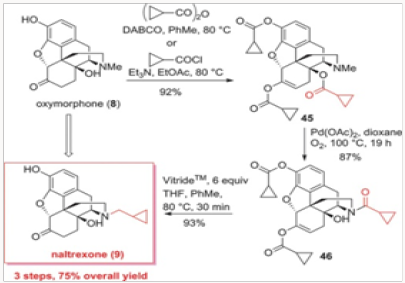
The palladium-catalyzed N-demethylation/acylation strategy was ultimately applied to a very efficient synthesis of naltrexone, as shown in Scheme 8. Oxymorphone 8 was peracylated with the anhydride derived from cyclopropylcarboxylic acid or the corresponding acyl chloride and subjected to the palladiumcatalyzed N-demethylation/acylation protocol. We have discovered that during the demethylation/acylation protocol the C-14 acyl group migrated to the N-17 site. The conditions previously used for this process required acetic anhydride. Thus, with the C-14 protected as acetate, no additional acyl donor was required. This was indeed fortuitous for several reasons. First, the overprotection of the phenol and the C-6 ketone was rather inexpensive, as the acyl group is both readily available and easily recyclable. Second, we have shown in separate experiments that the migration form C-14 to N-17 is intramolecular and therefore the N- demethylation of 45 could easily be performedin the presence of other acyl donors.10 Third, and very important, the vitride reduction of 46 leads to naltrexone and either cyclopropyl carboxylates or cyclopropylmethyl alcohols, both of which can be recycled and reused in eventual large-scale commercial applications. The final outcome was a three-step conversion of oxymorphone to naltrexone in a 75% overall yield (Figure 22-24).
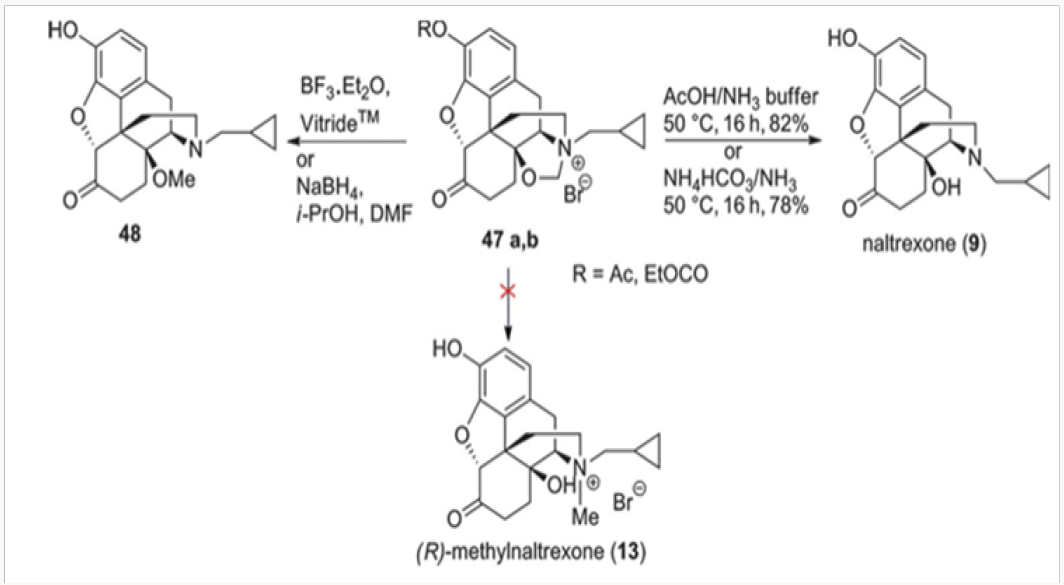
(R)-methylnaltrexone
From oxazolidines 42a and 42b
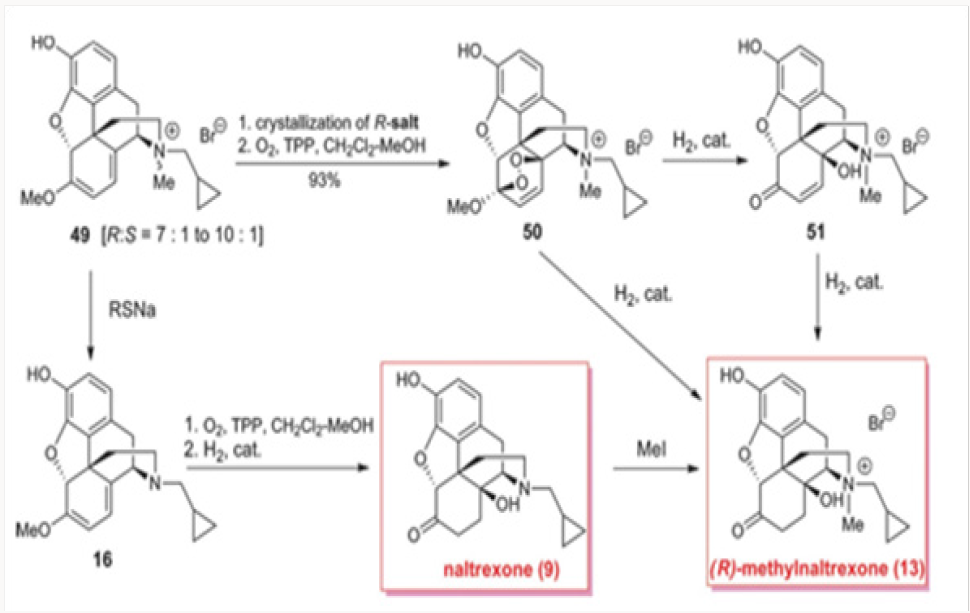
Our first attempt at the synthesis of methylnaltrexone 13 was based on the recognition that the methylene bridge in oxazolidines 42 was fixed in the (R)-configuration. We reasoned that the conversion of 42 to a cyclopropylmethyl salt 47 followed by a regioselective cleavage with a hydride may lead directly to methylnaltrexone, as depicted in Scheme 9. Unfortunately, hydride attack on the methylene bridge resulted only in the neutralization of the nitrogen providing the C-14 methoxy derivative 48. Under no conditions have we observed the tendency of the C-14 alkoxide to act as a leaving group. The hydrolysis of the salts 47a and 47b with con- comitant deprotection of the phenol provided for an additional synthesis of naltrexone 9.9By 1O2 cycloaddition to oripavine salts or cyclopropylmethyl nororipavine. The N-demethylation of the mixture of (R)- and (S)-cyclopropylmethyl salts 49 derived from oripavine was used to access cyclopropylmethyl nororipavine 16 during the initial approaches to u-prenorphine. The pure (R)-salt of 49 could be obtained by crystallization of the mixture and subjected to singlet oxygen cycloaddition to produce the endocyclic peroxide 50 (Scheme 10). Reduction of the peroxide, with the concomitant generation of C-6 ketone, yielded either enone 51 or, depending on the conditions, (R)-methylnaltrexone 13. The same cycloaddition of singlet oxygen was applied to 16 to yield naltrexone 9, after hydrogenation of the intermediate enone. Alkylation of naltrexone by literature methods provided (R)-methylnaltrexone 13.11 The two routes were com- parable in efficiency (Figures 25-29).
Naloxone
Alkylation of Noroxymorphone
The two distinct approaches for the replacement of the N-methyl group with other alkyl groups involved selective N-demethylation of salts or palladium-catallyzed N-demethylation/ acylation. Both worked well with simple alkyl groups but neither was applicable to the synthesis of naloxone. The nucleophilic demethylation of N-methyl-N-allyl salts occurred at the site of the allyl group. The palladium-catalyzed process required the use of acryloyl anhydride or the corresponding chloride and all of our attempts produced extremely complex mixtures. Thus the easiest route to naloxone was a simple alkylation of noroxymorphone 43, derived by hydrolysis of oxazolidines 42, with allyl bromide (Scheme 7) [9].
Grignard Additions to Oxazolidines
By far the best method for naloxone synthesis proved to be the nucleophilic opening of the oxazolidines with various Grignard reagents, as shown in Scheme 11. Our initial attempts at methylnaltrexone synthesis derived from the expectation that the carbon of the methylene bridge in oxazolidines 42 could be retained as the methyl group in methylnaltrexone. While that strategy was unsuccessful with the ammonium salts, we thought it would work well with the neutral oxazolidines. Indeed, protection of the C-6 ketone provided ketal 52, which was smoothly converted to the protected forms of naloxone, naltrexone, and nalbuphone by treatment with vinyl-, cyclopropyl-, or cyclobutyl Grignard reagents, re- spectively, in excellent yields. Hydrolysis of the ketals then furnished naloxone, naltrexone, and nalbuphone (Scheme 11). The entire sequence was eventually reduced to a one-pot operation. Slightly lower yields were obtained with the ethylene glycol derived ketals.12
The additional benefit of this strategy was a direct synthesis of nalbuphine 11 from oxazolidine 42a. We have prepared nalbuphine also by N-demethylation of N-cyclobutylmethyl quaternary salts derived from oripavine.13 Reduction of the C-6 ketone followed by the addition of cylobutylmagnesium chloride cleanly yielded nalbuphine 11. In all of these protocols, the acetateprotected phenol was freed with the use of excess Grignard reagent. The oxazolidine-based route to all of these opiate-derived agents proved to be general and very efficient.
Miscellaneous Projects
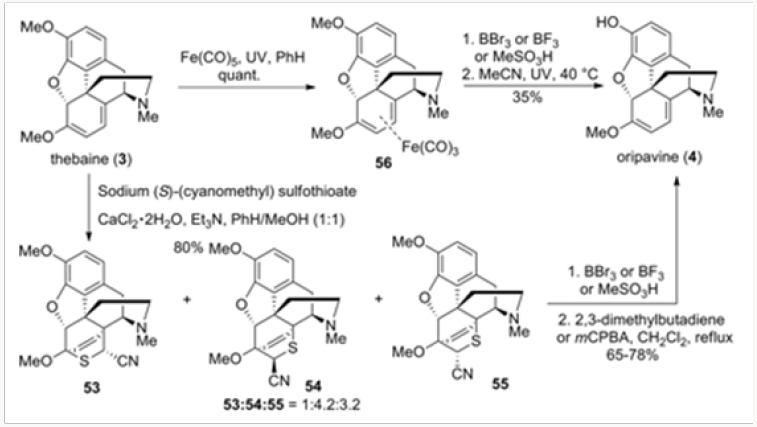
O-demethylation of Thebaine, also Known as Codeine Methyl Enol Ether, to Oripavine
Recently, we reported two methods for the conversion of thebaine to oripavine by O-demethylation. These methods were based on protection of the diene system in Thebaine, also known as codeine methyl enol ether, either as a complex with iron carbonyl or as a [4+2] cycloaddition adduct with a thioaldehyde. The outcomes of this study are abbreviated in Scheme 12. Both the iron carbonyl complex and the mixture of the Diels-Alder adducts were subjected to O-demethylation protocols with subsequent regeneration of the diene system. The overall yield of these protocols compared favorably with those of similar processes available in the literature [14].
Heteroatom Analogues of Hydrocodone
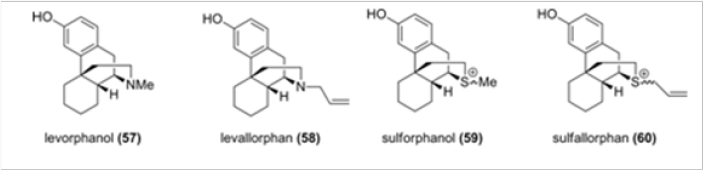
Snyder suggested that the configuration of the N-17 atom, be it nitrogen or another heteroatom, is responsible for agonist versus antagonist activity in morphinans and their truncated analogs such as levorphanol 57 and levallorphan 58 (Figure 4). 15 Lemaire replaced the nitrogen with a sulfonium salt functionality in sulforphanol 59 and sulfallorphan 60 assuming that the evaluation of the activities of these analogues would provide information about the relationship between the axial/equatorial configuration and the corresponding agonist/antagonist activity. Indeed, the agonist and antagonist activity was found to depend on the conformation of the S-substituent: the equatorial allyl group leads to antagonist activity and the axial methyl confers agonist activity.16 To our knowledge, there were no other heteroatom analogues of morphinans reported in the literature.
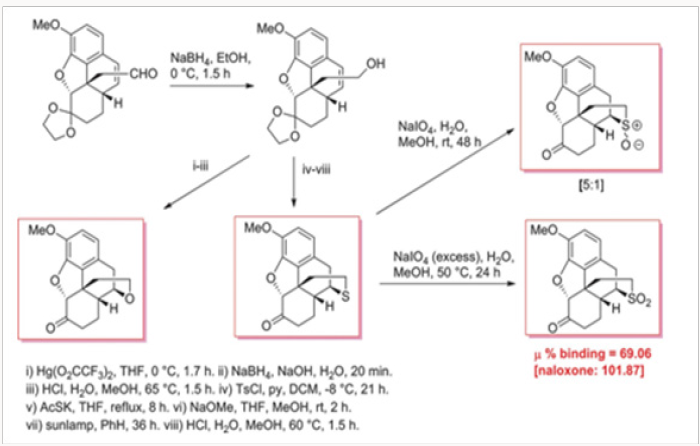
We have therefore chosen to study analogues of hydrocodone for which accurate biological data are available. In Scheme 13 is shown the synthesis of several heteroatom analogues of hydrocodone. Hydrocodone is converted, by Hofmann degradation, in a few steps to aldehylde 61, which is reduced to alcohol 62, the precursor of all heteroatom analogues. As shown in Scheme 13, the alcohol was converted to either oxo- or thio- analogues 63 and 64, respectively. Thioether 64 was also transformed to a mixture of sulfoxides 65 and also to sulfone 66.17 somewhat surprisingly, the only moderately active analogue was the sulfoxide 66, despite the fact that in this compound the axial/ equatorial position of the N-17 substituent is not relevant. This observation is in contradiction, at least for the heteroatom analogues of hydrocodone, with the endings reported for the sulfonium salts 59 and 60, in which the biological activities were related to the axial versus equatorial position of the substituent.
Conclusion
The HPLC method developed and validated allows a simple and fast quantitative simultaneous estimation of Naloxone Hydrochloride and Buprenorphine Hydrochloride from its formulation. All the validation parameters were found to be within the limits according to the ICH guidelines. The proposed method was found to be specific for the drugs of interest irrespective of the excipients present and the method was found to be simple, accurate, precise, rugged, and robust and can be involved in the routine analysis of the marketed formulation. The high percentage of stability under stress conditions confirms the suitability of the method. Therefore this method can be employed in quality control to estimate the amount of Naloxone Hydrochloride and Buprenorphine Hydrochloride in pure and pharmaceutical dosage forms. We have investigated several methods of N-demethylation of various morphinans. These methods were applied to process development toward the synthesis of buprenorphine, naltrexone, naloxone, nalbuphone, and nalbuphine. In each of these applications, a marked improvement was observed in the manufacture of these important medicinal agents. A comparison of the transition- metal versus enzymatic catalysis is shown in Figure 5. A detailed cost analysis will indicate which of the two routes more efficient for large-scale production is. Future endeavors should focus on the construction of transgenic organisms that would over express the cytochrome responsible for the N-demethylation. If successful, such an approach would be superior to all others, as the demethylation could be performed by fermentation in aqueous medium followed by a phase transfer alkylation to the final products. We should be able to report on further advances in this important area in due course
Biological Methods of N- and O-Demethylation
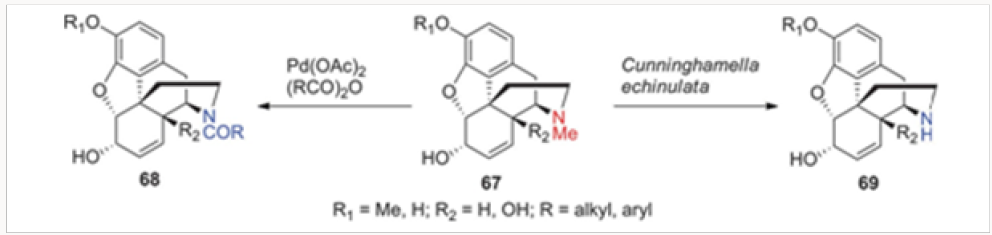
In addition to the palladium-catalyzed methods of N-demethylation, we have investigated biological means as well. The incubation of several morphine alkaloids and opiate-derived agents, depicted in Figure 4 as a general structure 67, with the fungus Cunninghamella echinulata led to isolation of the corresponding secondary amines of type 69.18 Surprisingly, the only morphinan that was immune to this process was morphine all other naturally occurring alkaloids as well as the commercially available opiates underwent a smooth demethylation. Presumably, a cytochrome enzyme within this fungus is responsible for the oxidative demethylation.
Read More About Lupine Publishers Journal of Food Please Click on Below Link:
https://lupine-publishers-food-and-nutrition.blogspot.com/




No comments:
Post a Comment
Note: only a member of this blog may post a comment.