Lupine Publishers| Journal of Drug Designing & Intellectual Properties
Abstract
The top priority in any health system is delivering of medicine as a strategic product. In the present context of a health-conscious society, management of pharmaceutical supply chains has become more complex because it involves the life-saving interest of human being and requires the participation of different stakeholders such as pharmaceutical manufacturers, wholesalers, distributors, customers, information service providers and regulatory agencies. Limited research is available in the area of pharmaceutical supply chains. Pharmaceutical companies, a most important player of the drug supply chain, are subject to many risks. These risks interrupt the quantity and quality of supply of medicine and their delivery to the accurate place and customers and at the correct time.
Keywords: Drug Counterfeiting; Temperature Controlled Logistics; Supplier Qualification; Performance Management; Globalization; Sustainability
Abbrevations: OTC: Over the Counter; CDSCO: Central Drug Standards and Control Organization; NPPA: National Pharmaceutical Pricing Authority; RFID:Radio Frequency Identification Technology; EPC: Electronic Product Code; EPC: Electronic Product Code
Introduction
Supply chain management is defined as the amalgamation of key business processes across the supply chain for the rationale of generating value for customers and stakeholders. Indeed supply chain management integrates supply and demand within and across companies in an efficient business model. [1,2] The Council of Supply Chain Management Professionals defines supply chain management as planning and management of all activities involved in sourcing, procurement, conversion and all logistics activities. There are a variety of aspects of evaluating in the supply chain; eliminating bottlenecks, balancing between tiniest material cost and transportation, optimizing manufacturing flow, maintaining the right mix and location of factories and warehouses, vehicle routing analysis, dynamic programming and efficient use of capacities, inventories, and labors are of main aspects of supply chain optimization [3, 4]. All stockholders need to institute the right configuration and adaptability to create best practice and to overcome the obstacles in continues changing environment. Pharmaceutical supply chain should provide medicines in the right quantity, with the acceptable quality, to the right place and customers, at the right time and with optimum cost to be consistent with health system's objectives and also it should make benefits for its stockholders. Supply chain is a set of players, processes, information, and resources which transfers raw materials, and components to finished products or services and delivers them to the customers. It includes suppliers, intermediaries, third-party service providers and customers. It also includes all of the logistics activities, manufacturing operations and activities with and across marketing, sales, product design, finance and information technology [5-7].
Benefits of Supply Chain Management
Supply chain management in the pharmaceutical industry can renovate the organization to make better use of assets and resources, to engender profits, to boost shareholder value, and to optimistically respond to customer demand. Effective supply chain management can effect and develop virtually all business processes, such as data accuracy, operational complexity reduction, supplier selection, purchasing, warehousing and distribution. Other benefits include [8]:
a) Quicker customer response and fulfilment rates
b) Shorter lead time
c) Greater productivity and lower costs
d) Reduced inventory supply throughout the chain
e) Improved forecasting precision
f) Fewer suppliers and shorter planning cycles
The Pharmaceutical Supply Chain
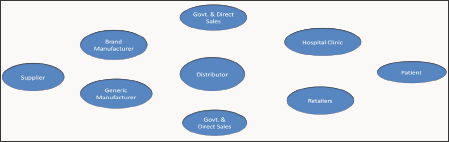
After a drug is launched, a completely different set of objectives, drivers, and constraints become dominant. The key stakeholders in Main Issues Related to Pharmaceutical Supply Chain final product. The Pharmaceutical Supply Chain this supply chain include multiple government agencies, hospitals, clinics, drug manufacturers, drug distributors, pharmacy chains, retailers, research organizations, and the FDA. To compound matters further, the same supply chain is responsible for the distribution of prescription drugs, over-the-counter (OTC) medicines, generics, as well as biologics having different handling needs and operational objectives. Indeed, there are numerous other organizations, such as insurance companies, healthcare management organizations, and GPOs, that further increase the complexity. Due to very different business objectives, these organizations make the task of managing supply chain all the more difficult. Furthermore, due to the regulatory nature of the industry and numerous merger and acquisitions to acquire more R&D expertise, many pharmaceutical supply networks have grown in an uncontrolled fashion rather than being planned for optimal performance [9] (Figure 1).
Figure 1: The Pharmaceutical Supply Chain.
Main Issues Related to Pharmaceutical Supply Chain
i. Issues related to Counterfeiting.
ii. Unfavorable reaction of the drug to the patients.
iii. Issues rose due to entities of supply chain operations.
iv. Manufacturing issues like mixing incorrect input raw materials, or cross contamination due to manufacturing more than one drug in the same facility, or improper labeling of the final product.
v. Retailer's issues including improper temperature controls and handling.
vi. Transportation issues caused by mishandling, improper temperature controls, and the use of improper shipping mode.
vii. Storing and warehousing issues such as using improper temperature controls, improper handling in the warehouse and mixing products with raw materials.
Figure 2: Strategic issues in pharmaceutical supply chain.
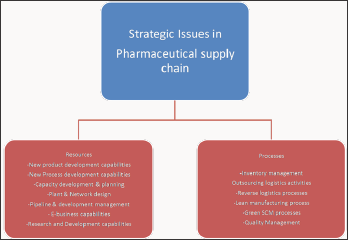
viii. Raw material suppliers issues like improperly prepared raw material, raw material with high impurity levels and mislabeling of raw material shipments (Figure 2).
The Regulatory System
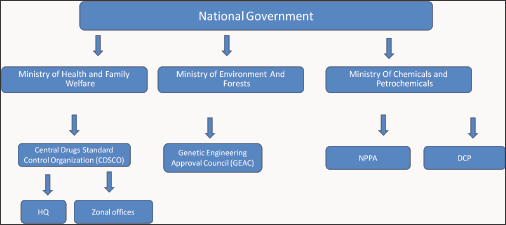
The regulatory system of the Government of India has a federal form that divides the medical regulatory system into national and state authorities. The principal regulatory bodies that are answerable for the approval, production, and marketing of quality drugs in India are the following entities [10]:
a. Central Drug Standards and Control Organization (CDSCO): This agency sets standards to ensure the safety and quality of drugs, diagnostics, cosmetics, devices, and supervision of the importation of drugs.
b. National Pharmaceutical Pricing Authority (NPPA): This agency fixes or revises the prices of decontrolled bulk drugs.
c. Ministry of Chemicals and Petrochemicals: This organization supervises pharmaceutical sector policy, planning, development, and regulatory activities pertaining to the chemicals, petrochemicals (Figure 3).
Figure 3: Regulatory system of government of India.
Temperature Controlled Logistics
India is a vast continent with different temperatures and environmental conditions varying 25 to 500c at different places. This presents a whole different set of challenges for the drug manufacturers to ensure drugs are maintained at the requisite temperatures throughout their lifecycle. The problem of temperature controlled logistics is one of the common noteworthy problems. The failure to maintain drugs at their prescribed temperature often results in the loss of efficacy. The drugs that require stringent cold storage and transport are often expensive and are often targeted by the drug counterfeiters due to their high value.
Radio Frequency Identification Technology (RFID) and Electronic Product Code (EPC) and its Effect on The Pharmaceutical Supply Chain [11, 12]
A RFID system fundamentally consists of four items, a host computer, readers, encoders and tags. Tags are manufactured of a microchip, size is approx 0.2 mm or 0.4 mm and a bendable antenna entrenched in a plastic-coated inlay having numerous forms and dimensions depending on the context and performance required. The information can be written onto the tags by an encoder printer, which is consequently read by a reader that converts the electromagnetic wave pattern coming from the tag into digital signals and transmits them to the information system by a terminal computer. Data are stored into the tag chip in the form of an Electronic Product Code (EPC).
Read More About Lupine Publishers Journal of Drug Designing & Intellectual Properties Please Click on Below Link: https://lupine-publishers-drug-designing.blogspot.com/

No comments:
Post a Comment
Note: only a member of this blog may post a comment.