Lupine Publishers| Journal of Nanomedicine
Abstract
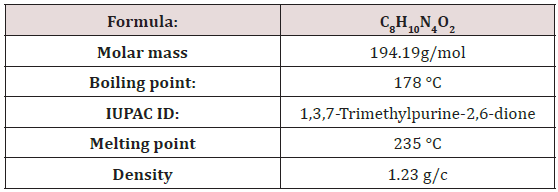
This investigation was carried out to determine the pH, levels of caffeine contents in five soft drinks and four energy drinks available in local market of Pakistan. pH were measured by pH meter. Quantitative estimation of caffeine was performed by a simple and fast standard UV spectrophotometric method (Perkin Elmer lambda 35 UV/Vis spectrometer) using carbon tetrachloride as the extracting solvent at 270nm wave length. The minimum caffeine level of soft drinks was observed in Brand-3 (10.69 mg/serving), while Brand-5 showed the highest caffeine content (42.17mg/serving) showing a range from 10.6 to 42.17mg/serving. The levels of caffeine in all energy drink samples are well below the maximum allowable limits set by the food regulatory bodies, except E2. The E2 has greater calculated concentration then the labeled concentration .The minimum caffeine level of energy drinks was observed in E4 (32.04mg/L) while E2 showed highest caffeine level in energy drinks (101.705mg/serving) showing range from 32.04mg/ serving to 101.705mg /serving . And the pH ranges of these soft drinks were (2.29 to 3.02) and in energy drinks (2.85 to 3.28) .Further investigations are needed to determine the suitability of these soft and energy drinks.
Keywords: Caffeine, Cocoa Beans, UV Spectrophotometer, Comparison of Soft And Energy Drinks, White Crystalline Xanthine Alkaloid, Anthropologists, Warding off Drowsiness, Restoring Alertness , Psychoactive Substance, Decaffeinated Coffee
Introduction
Caffeine is a common ingredient of energy drinks. It is deliberately added as a flavoring agent and to make the drinks addictive [1]. Caffeine is a bitter, white crystalline xanthine alkaloid that acts as a psychoactive stimulant drug and a mild diuretic. Around sixty plant species are known to contain caffeine [2]. Common sources are the “bean” (seed) of the coffee plant; in the leaves of the tea bush; and in kola nuts. Other sources include yaupon holly leaves, South American holly yerba mate leaves and seeds from Amazonian maple guarana berries [3]. Columbia In 1819, the German chemist Friedrich Ferdinand Runge isolated pure caffeine for the first time [4]. Caffeine is one of the world’s most widely used drugs. Many anthropologists believe its use may date back to the Stone Age. Caffeine was first extracted from coffee in 1821 [5]. Caffeine is a naturally occurring substance found in the leaves, seeds or fruits of over 63 plants species worldwide and is part of a group of compounds known as methyl xanthines. The most commonly known sources of caffeine are coffee, cocoa beans, cola nuts and tea leave [6]. Caffeine is a naturally occurring substance found in humans, caffeine is a central nervous system (CNS) stimulant [7]. It has theeffect of temporarily warding off drowsiness and restoring alertness. Beverages containing caffeine, such as coffee, tea, soft drinks and energy drinks, enjoy great popularity [8]. Caffeine is the world’s most widely consumed psychoactive substance. Adults receive nearly three quarters of their daily caffeine from coffee. Children receive one half of their caffeine from soft drinks.
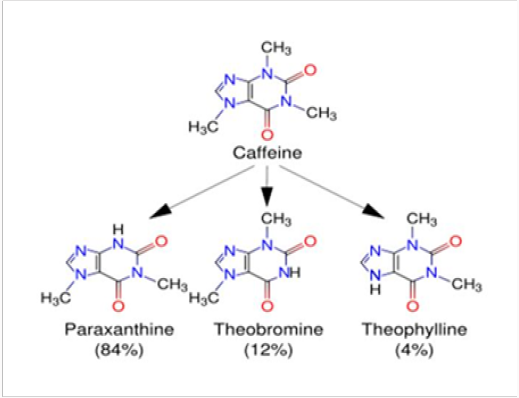
Energy drinks represent a fast-growing beverage market (Table 1). Energy drinks vary in the amount of caffeine can range from around 50-300mg. Most people experience no behavioral effects with less than 300 mg caffeine. Sleep is more sensitive and can be disrupted by 200mg caffeine [9]. The caffeine content in your average cup of coffee is around 100mg. Decaffeinated coffee isn’t actually caffeine-free, and can contain up to 12mg of caffeine. Your average cup of tea contains 85mg of caffeine. A single can of commercially available energy drink can have anywhere between 80 and 280mg of caffeine depending on the can size. Green tea is close behind with 60mg of caffeine, followed by white tea with 55mg. Slim-fast chocolate drinks come in at 20mg of caffeine in a single serving [10]. Caffeine is metabolized in the liver into three primary metabolites: paraxanthine (84%), theobromine (12%), and theophylline (4%) [11] Caffeine is metabolized in the liver by the cytochrome P450 oxidase enzyme system (specifically, the 1A2 isozyme) into three metabolic dimethyl xanthines which each have their own effects on the body [12] (Figure1). Paraxanthine (84%) Increase free fatty acid levels in the blood plasma. Theobromine (12%) increases urine volume. Theophylline (4%) Relaxes smooth muscles of the bronchi, and is used to treat asthma [13]. An acute overdose of caffeine, usually in excess of 250 milligrams (more than 2-3 cups of brewed coffee), can result in a state of central nervous system overstimulation called caffeine intoxication [14]. The effects of caffeine on the body may begin as early as 15 minutes after injecting and last up to hours [15]. Caffeine is highly addictive, caffeine increase stress level, caffeine accelerates aging and wrinkles [16]. Caffeine intake of 150-300mg after a 10h fast increased urinary calcium excretion 2-3h after exposure in adolescent men and women [17]. Dehydration is a major drawback of caffeine consumption, and results from the drugs ability to increase urine production. In addition to dehydration, caffeine causes some people to get jittery stomachs or “coffee stomach” - which can be quite uncomfortable and mask any potential benefits [18]. 100-200mg dose of caffeine result in increased alertness and wakefulness, faster and clearer flow of thought, increased focus, and better general body coordination [19]. Caffeine makes people more alert, less drowsy, and improves coordination. Combined with certain pain relievers or medicines for treating migraine headache [20]. In a large 217,883 person study, those that consumed caffeine from any source had less kidney stone formation than those that did not consume caffeine [21].
Aims
I selected this topic determination of caffeine in drinks available in market because now a days energy and soft drinks play vital role in our daily life and become necessity of our life that’s why I want to know all benefits and disadvantages of these drinks .so I determined the amount of caffeine in 9 brands of soft and energy drinks by using UV/VIS Spectrophotometer to obtained practical knowledge in the use of basic UV/VIS Spectrophotometer equipment. The main purpose of this research is to raise the awareness of negative effects of caffeine on human health because many companies sales their products by highlighting few advantages of caffeine so companies should be labeled the caffeine content in drinks and also its effects as well as benefits.
Literature
Guzin Alpdogan concluded the caffeine concentration range in coca cola is 149.32±0.68 mg/ml by using derivative spectrophotometric method and use Philips 8740UV/VIS Spectrophotometer at 232.7-245.2nm and the caffeine concentration range of coffee is 1.36 ±0.03 percent at 268.5-289.5nm and in the tea range of caffeine concentration is the 1.53 ± 0.03percent at 286.0-300.0nm. Ahmad H. Alghamdi determined the content levels of some food additives (Aspartame, Caffeine, Sodium benzoate) in 29 different beverage samples commercially available in Riyadh local markets by using UV spectrophotometric method ( Perkin-Elmer, USA).The caffeine contents in energy drink samples ranged from 22.64ppm to 34.96ppm. H.N Wanyika discussed the levels of caffeine in certain coffee (nescafe, africafe, dormans) and tea (chai maramoja, kericho gold, sasini, finlays premium) brands found in the Kenyan market were determined using High performance liquid chromatography (HPLC) and UV/ Vis spectrophotometer (Shimadzu) at 274nm which gave a concentration of 471 .73 ± 1 96.92 ppm. KapilKalra studied the concentration of Caffeine of seven brands of soft drinks with the use of an analytical method, U.V Spectrophotometer Shimanzu 1800 Compact. Which will tell us the best brand amongst different brands containing caffeine at 271.2nm , highest concentration of caffeine was found in Power-ex (46 μg/ml) and the lowest concentration of caffeine in XXX (19.5μg/ml). Sarmad G. Mohammed has done research on ten brands of beverages (soft and energy drinks) consumed in Basrah governorate/Iraq, and he were determinate its pH, trace minerals and caffeine contents by using UV/VIS spectrophotometer (shimadzu AA 630-12) at 254nm wavelength. The caffeine concentration in beverages are these Kal aschnikow 103.13±1.14, 2 Boom Boom 102.56±1.11, Power horse 94.53±0.10 ,O290.89±1.02, Pit bull 79.99±1.00 , Pepsi 80.00±1.34 ., Wild tiger 79.94±0.22 , Mountain dew 44.08±0.34.
Magut Hillary studied the different brands of soft drinks and juices were randomly sampled from different stores in Eldoret town, the caffeine levels were found to be in the range of 1.43mg/L and 40.51mg/L. Muhammad Mufakkar has done research on the eight brands of soft drinks and determine the caffeine concentration by using Ultraviolet spectroscopy at 272nm [22]. The highest concentration of caffeine was found in Sting 500mL (560.29μg/ mL). The lowest concentration of caffeine was found in 7up 500mL (29.71μg/mL). Mozammel Hossain investigated to carry out to determine the pH, levels of caffeine and reducing sugar contents in five energy drinks available in local market in Rajshahi, Bangladesh by using U.V/VIS Spectrophotometer the pH of the beverages was perfectly acidic ranging from 2.85 to 3.11. The minimum caffeine level was observed in Brand-4 (40.34mg/serving), while Brand-5 showed the highest caffeine content (244.57 mg/serving) showing a range from 40.34 to 244.57mg/serving.
Material and Methods
Instrument
UV/VIS spectrometer Perkin Elmer lambda 35. The double beam spectrophotometer having the range 190-1100 nm and bandwidth: 0.4-4nm (variable).
pH Determination
Beverages PH were determined by using Sartorius pH meter.
Preparation of Stock Solution
i. All glassware was washed with distilled water.
ii. Then glassware was dried in oven at 105 degree Celsius.
iii. A 100ppm stock standard of caffeine was prepared by dissolving 20 Mg caffeine in 250ml
carbon tetra chloride in 200ml volumetric flask.
Preparation of Standard Solution
Working standards were prepared by pipetting 0.1, 0.2, 0.3, 0.4, 0.5ml respectively aliquots of stock standard solution into separate volumetric flasks of 100ml and dilute it with carbon tetrachloride and forms 10, 20, 30, 40, 50mg/L standards solution. The absorbance of each solution was measured at absorption maximum of 270nm using 10mm quartz cuvette.
Caffeine Extraction Procedure
i. The brands of soft and energy drinks were taken by different shops.
ii. Then the sodium carbonate solution is prepared by dissolving 20g sodium carbonate into distilled water in 25ml volumetric flask.
iii. Then separating funnel was taken and adjust it in the stands with beakers.
iv. Then 5ml of drink sample was drawn in the separating funnel by addition of distilled water and add 1ml of sodium carbonate solution in the separating funnel and add 20ml of carbon tetrachloride in it.
v. The caffeine was extracted by inverting funnel at least three times venting the funnel after each inversion.
vi. The non aqueous carbon tetra chloride layer was removed to a clean 50ml volumetric flask.
vii. Another 20ml portion of carbon tetra chloride was added to aqueous solution in separating funnel and extraction procedure was repeated twice and carbon tetra chloride layers combined.
This procedure was repeated for all drink samples the absorbance of resulting solutions was measured on UV/Vis Spectrophotometer at 270nm using 10mm quartz cuvette.
Results and Discussion
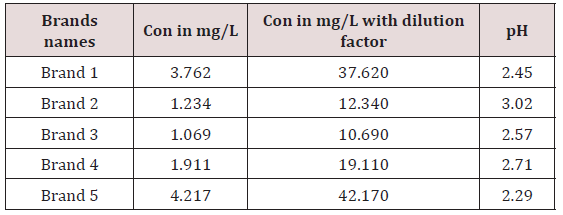
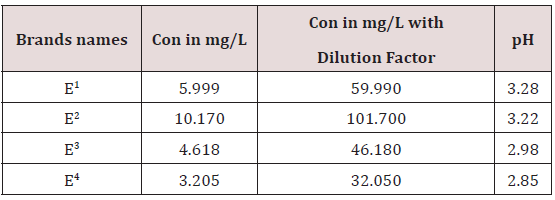
The regression line is Y=0.036x-0.0112. Dilution factor= flask volume / sample volume =50/5=10.
Caffeine Concentration and pH
The main objective of this research is to know the caffeine level in soft and energy drinks high or low then the published value or the FDA recommended value. The Brand 2 has the highest pH 3.02 values among all soft drinks so it means it is less acidic among all soft drinks .And Brand 5 has lowest pH 2.29 among all soft drinks it means it is more acidic among all soft drinks .The soft drinks having pH range 2.29 to 3.02. The ideal (neutral) pH of the mouth ranges from 6.5-7.5.A pH of 5.5 is considered to be the threshold level for the development of dental decay. Both soft drinks and sports drinks have been shown to have a pH between 2.5 and 3.5. Demineralization of tooth enamel will occur more rapidly if the pH drops below the critical 5.5 level for long periods of time and if the pH is dropped below the critical level frequently. All kinds of soft drinks are acidic and that cola drinks especially make our bodies poor in oxygen.
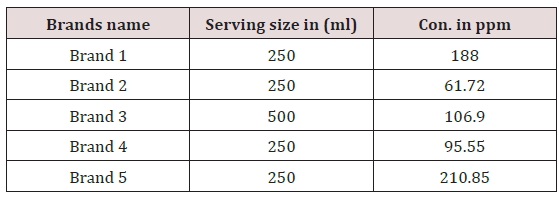
The caffeine concentration range in soft drinks is 10.69- 42.17ppm. And the concentration of Brand 1 is 37.62 at 270nm .Similarly caffeine concentration in Brand 2 was found to be 12.345ppm,caffeine concentration in Brand 3was found to be 10.69ppm,caffeine concentration in Brand 4 was found to be 19.11ppm and caffeine concentration was found to be 42.17ppm. The highest caffeine concentration was found to be in Brand 5 which is 42.17ppm and it has lowest pH value so it means Brand 5 is most acidic among all soft drinks .so it is strongest central nervous system stimulant .so it can be discontinued in market. The lowest caffeine concentration was found to be in Brand 3 which is 10.69ppm and pH values are high so it means these brands are less acidic so it can be sold in market and a weak central nervous system stimulant .The US food and Drug Administration (FDA,2006) limits maximum amount of caffeine in soft drinks is 200pm in 6mg/oz .therefore caffeine content allowed in soft drinks may be in range between 30-72mg in 355ml (Table 2).
Statistical Analysis of Soft Drinks
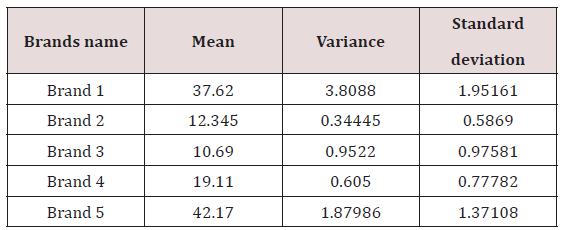
The variance and standard deviation of Brand 1 was 3.8088 and 1.95161 respectively .The variance and standard deviation of Brand 2 was 0.34445 and 0.5869 respectively. The variance and standard deviation of Brand 3 was 0.9522 and 0.5869 respectively. The variance and standard deviation of Brand 4 was 0.605 and 0.77782 respectively. The variance and standard deviation of Brand 5 was 1.87986 and 1.37108 respectively.
Mean = Sum of X values / N(Number of values)
Standard deviation = S=√ [ (X-M) ] ^2/n-1
Variance = s2.
Caffeine Concentration and pH
The pH range in energy drinks is 2.85 _3.28 .The Brand 9 having pH 2.85 it means it is highest acidic among all energy drinks and E1 have highest pH which is 3.28 so it means it less acidic among all energy drinks. The low pH values could be as a result of presence of carbon dioxide, phosphoric acids, malic acid, tartaric acid used as preservatives by manufactures of these beverages. The acids inhibit growth of microorganism, bacteria, fungal may contaminate beverages. E4>E3>E2> E1.The caffeine concentration range in energy drinks are 32.05-101.905ppm and the concentration of E1 59.95ppm .Similarly caffeine concentration in E2 was found to be 101.705ppm,caffeine concentration in E3 was found to be 46.185ppm,caffeine concentration in E4 was found to be 32.05ppm . The highest caffeine concentration was found to be in E2which is 101.705ppm and it has lowest pH value so it means E2 is most acidic among all energy drinks .so it is strongest central nervous system stimulant .so it can be discontinued in market. The lowest caffeine concentration was found to be in E4which is 32.05ppm and pH values are high so it means these brands are less acidic so it can be sold in market and a weak central nervous system stimulant (Table 3).
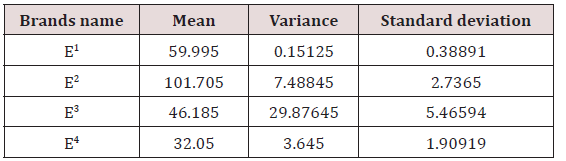
Statistical Analysis of Energy Drinks
The variance and standard deviation of E1 was0.15125 and0.38891 respectively. The variance and standard deviation of E2 was 7.48845 and2.7365 respectively. The variance and standard deviation of E3 was 29.87645 and 5.46594respectively. The variance and standard deviation of E4 was3.645 and1.90919 respectively (Table 4).
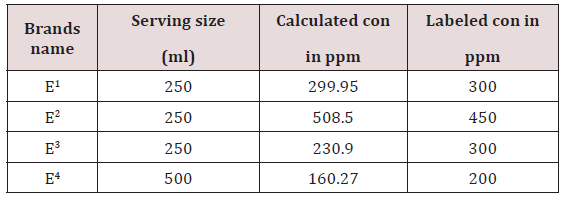
Comparison of Labeled and Calculated Concentrations
The E1 had been labeled concentration was 300mg per 250ml and calculated 299.95mg per 250ml .The E2 is the first brand which have ginseng that cause low tension , relieve stress, Stimulate metabolism. It is manufacture by king beverages and its calculated caffeine content is 508.5mg per 250ml and labeled concentration was 450ppm per 250ml. The E3 had labeled concentration was 300mg per 250ml and calculated 230.9mg per 250ml so it is less than its labeled caffeine content amount .The E4 had labeled concentration was 200mg per 500ml and calculated amount of caffeine 160.27mg per 500ml it is less than its labeled caffeine content amount (Table 5-7).
Concentration in ppm 250ml =concentration in ppm per 5ml *50.
Concentration in ppm per 500ml= concentration in ppm per 5ml *100.
Conclusion
Determination of caffeine content in non alcoholic beverages and energy drinks is very important analytical process safeguard the well being of people who are unaware to adverse effects of caffeine. In soft drinks the Brand 5 have highest concentration of caffeine that is 42.17ppm and Brand 3 having low concentration of caffeine 10.69ppm and in energy drinks Brand 2 having high concentration of caffeine that is 101.705ppm (Figure 2). Brand 9 having low concentration of caffeine that is 32.05ppm among all energy the process of determination of caffeine in drinks can done by many analytical method but in this research UV/VIS Spectrophotometer was used because it is relatively easy, fast, cheap ,highly sensitive and give accurate concentration of caffeine.
Read More about Lupine Publishers Journal of Nanomedicine Please Click on Below Link: https://lupine-publishers-nano-science.blogspot.com/

No comments:
Post a Comment
Note: only a member of this blog may post a comment.