Lupine Publishers| Online Journal of Neurology and Brain Disorders (OJNBD)
Abstract
In this study we have analyzed 30 people. 10 patients Wolfram syndrome and 20 persons control group. The genes WFS1 and CISD2, analyzed in terms of genetic mutations made. In this study, people who have genetic mutations were targeted, with nervous disorders Wolfram syndrome. In fact, of all people with Wolfram syndrome. 10 patients Wolfram syndrome had a genetic mutation in the genes WFS1 and CISD2 Wolfram syndrome. Any genetic mutations in the target genes control group did not show.
Keywords: Genetic study; Wolfram syndrome; Mutations The gene WFS1 and CISD2; RT-PCR.
Generalizations of Wolfram Syndrome
Wolfram syndrome is a genetic disorder that affects many body systems. The specific characteristics of Wolfram syndrome include: high blood sugar levels due to insulin hormone deficiency (diabetes mellitus) and progressive vision loss due to degeneration of the nerves that transports visual information from the eye to the brain (vision atrophy).
Symptoms and Symptoms of Wolfram Syndrome. People with Wolfram syndrome often reveal pituitary gland disorders that lead to excessive urination, hearing loss caused by changes in the inner ear (sensory nervous system depression), problems with the urinary tract, decreased testosterone levels in men (hypogonadism), Nervous or psychiatric disorders [1].
Diabetes mellitus is usually the first symptom of Wolfram syndrome, which is commonly diagnosed at about 6 years of age. Almost all people with Wolfram syndrome who have diabetes need alternative insulin therapy. Sighted atrophy is the second symptom of Wolfram syndrome, which usually occurs at the age of 11 years. The first symptoms of visual atrophy are vision loss and peripheral vision. Over time, vision problems get worse, and people who have vision atrophy usually lose their sight completely, about eight years after the onset of the first signs of atrophic vision [2].
Figure 1: A schematic of the cause of Wolfram syndrome and common diseases in it.

The pituitary gland, located at the top of the brain, is thickened in diabetes and does not function naturally. The disorder also releases a hormone called vasopressin, which helps control your body's water balance and urine output. Approximately 70% of people with Wolfram syndrome have diabetes. Pituitary gland dysfunction can also lead to hypogonadism in affected men. Testosterone deficiency that occurs with hypogonadism also affects sexual development and development. About 65 percent of people with Wolfram syndrome have sensory inferiority that can vary from depression to birth to mild hearing loss during adolescence, which worsens over time (Figure 1).
About 60% to 90% of people with Wolfram syndrome have urinary tract problems. Urinary problems include blockage of the canals between the kidneys and the bladder, large bladder that can not normally be drained (high capacity bladder), urinary tract distention (bladder sphincter dissonance), and lack of urinary flow control (urinary incontinence) [3]. About 60% of people with Wolfram syndrome experience neuromuscular or psychiatric disorders, most of them with imbalance (Ataxia), which usually begins early in puberty. Other neurological problems experienced by people with Wolfram syndrome include irregular breathing due to the inability of the brain to control respiration (central apnea), loss of sense of smell, loss of oral reflexes, muscle spasm (myoclonus), seizure, decrease Feeling in the lower limbs, such as the legs (peripheral neuropathy) and mental disorder. Mental disorders in people with Wolfram syndrome include severe depression and aggressive behaviours [4].
Up to now, 2 types of Wolfram syndrome have been identified, the first type has symptoms that are mentioned, and the second type has gastric ulcer or intestinal ulcer with excessive hemorrhage after injury. Therefore, the tendency to excessive hemorrhage associated with ulcers usually results in abnormal bleeding in the digestive system. It is worth noting that Wolfram syndrome, often due to some diseases related to diabetes and neurological problems, often leads to mortality in the middle of adulthood [5].
The Cause of Wolfram&s Syndrome
The mutation in the WFS1 gene, which is based on the short arm of chromosome 4 as 4p16.1, causes more than 90% of Wolfram type 1 syndrome. This gene provides instructions for the synthesis of a protein called the Wolfram in, which regulates the amount of calcium in the cells. The balance of calcium is important for many different cell functions, including cell-to-cell communication, muscle contraction, and protein processing. Wolfram in protein is found in many different tissues such as pancreas, brain, heart, bones, muscles, lungs, liver and kidneys. Within the cells, wolf amine is present in the cell membrane called endoplasmic reticulum, which is involved in the production, processing and transport of proteins. Wolfram in function in the pancreas, where the protein helps to process protein processing called pransul in to become an adult insulin hormone. This hormone controls blood glucose.
Figure 2: Schematic view of chromosome number 4 where the WFS1 gene is based on the short arm of this chromosome as 4p16.1

Therefore, WFS1 gene mutations result in the production of a wolf amine protein whose function is diminished or does not function properly. As a result, calcium levels in the cells are not regulated and the endoplasmic reticulum does not work properly [6,7] (Figure 2).
When the endoplasmic reticulum does not exhibit sufficient function of the vlfremin, the cell suffers from a physiological cell death (apoptosis). The death of pancreatic cells, especially the cells that produce insulin (beta cells), causes diabetes in people with Wolfram syndrome. The gradual destruction of the cell along the visual acuity ultimately leads to blindness in people with Wolfram syndrome. Deaths of cells in other body systems may cause symptoms and symptoms of Wolfram type 1 syndrome.
A specific mutation in the CISD2 gene, which is based on the long arm of chromosome 4, 4q24, causes Wolfram type 2 syndromes. The CISD2 gene provides instructions for protein synthesis that is located in the outer membrane of cell structures called mitochondria. As you know, the mitochondrial organ is the cell's energy production center. The exact performance of the CISD2 protein is unknown, but it is believed that this protein helps the mitochondria to function normally [8] (Figure 3).
The mutation in the CISD2 gene, which causes Wolfram type 2, produces an abnormal, non-functional, abnormal CISD2 protein, which results in the mitochondrial organ not functioning correctly and does not generate the energy required for cells. Therefore, cells that do not receive enough energy will not have enough energy to function properly and will eventually die. High-energy cells, such as neural cells in the brain, the eye, or the digestive tract, are most susceptible to death due to energy loss. It is not yet clear why people with Wolfram syndrome type 2 with the CISD2 gene mutation also experience wound and bleeding problems in addition to the usual Wolfram disease. It should be noted that some patients with Wolfram syndrome have no mutations in the WFS1 and CISD2 genes, and the cause of the syndrome in these patients is unknown. But other genes may also be involved in Wolfram syndrome that has not yet been identified [9].
Figure 3: Schematic view of chromosome number 4, in which the CISD2 gene is located in the long arm of this chromosome 4q24.

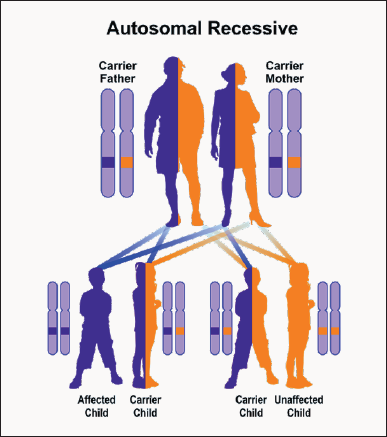
Wolfram syndrome type 1, caused by the mutation of the WFS1 gene, follows an autosomal recessive hereditary pattern. Therefore, for the creation of Wolfram type 1 syndrome, two versions of the mutated gene of WFS1 (one parent and one of the mother) are needed, and the chance of having a child with Wolfram type 1 in this case is 25% for each pregnancy. Type 2 Wolfram syndrome, caused by the mutation of the CISD2 gene, also follows an autosomal recessive hereditary pattern. Therefore, for the creation of Wolfram type 2 syndrome, two versions of the mutant gene called CISD2 (one parent and one mother) are needed, and the chance of having a child with Wolfram type 2 in this case can be 25% for each pregnancy [10] (Figure 4).
Figure 4: A schematic view of the autosomal-inheritance inheritance pattern that Wolfram Syndrome also follows with this mutation in the WFS1 and CISD2 genes.
Materials and Methods
In this study, 10 patients with Wolfram Syndrome and 20 persons control group were studied. Peripheral blood samples from patients and parents with written permission control were prepared. After separation of serum, using Real Time-PCR technique of tRNA molecules was collected. To isolate Neuroglial cells erythrocytes were precipitated from hydroxyethyl starch (HES) was used. At this stage, HES solution in ratio of 1 to 5 with the peripheral blood of patients and controls were mixed. After 60 minutes of incubation at room temperature, the supernatant was removed and centrifuged for 14 min at 400 Gera. The cell sediment with PBS (phosphate buffered saline), pipetazh and slowly soluble carbohydrate ratio of 1 to 2 on ficole (Ficol) was poured in the 480G was centrifuged for 34 minutes. Mono nuclear Neuroglial cells also are included, has a lower density than ficole and soon which they are based. The remaining erythrocytes have a molecular weight greater than fico eland deposited in test tubes. The supernatant, which contained the mono nuclear cells, was removed, and the 400 Gera was centrifuged for 12 minutes. Finally, the sediment cell, the antibody and Neuroglial cells was added after 34 minutes incubation at 5°C, the cell mixture was passed from pillar LSMACS. Then the cells were washed with PBS and attached to the column LSMACSS pam Stem cell culture medium containing the transcription genes WFS1 and CISD2, and were kept. To determine the purity of Neuroglial cells are extracted, flow cytometry was used. For this purpose, approximately 4-5 x 103 Neuroglial cells were transfer red to1.5ml Eppendorf tube and then were centrifuged at 2000 rpm for 7 minutes at time. Remove the supernatant culture medium and there maiming sediment, 100μl of PBS buffer was added. After adding 5-10μl PE monoclonal anti body to the cell suspension for 60 min at 4°C, incubated and readimme diately by flow cytometry. For example, rather than control anti body Neuroglial cells PE, IgG1 negative control solution was used.
i. Total mRNA extraction proceeds urein cludes 1ml solution spilled Qiazolon cells, and slowly and carefully mixed and incubated at room temperature for 5 minutes. Then 200μl chloroform solution to target mix, and then transfer the micro tubes was added, and the shaker well was mixed for 15 seconds. The present mix for 4 minutes at room temperature and then incubated for 20 min at 4°C on was centrifuged at 13200 rpm era. Remove the upper phase product was transfer reeducates new micro tube and to the one times the volume of cold ethanol was added. The resulting mixture for 24 hours at -20°C was incubated.
ii. Then for 45 min at 4°C on was centrifuged at 12000 rpm era. Remove the super natant and the white precipitate, 1ml of cold 75% ethanol was added to separate the sediment from micro tubes were vortex well. The resulting mixture for 20 min at 4°C on by the time we were centrifuged 12000 rpm. Ethanol and the sediment was removed and placed at room temperature until completely dry deposition. The precipitate was dissolved in 20μl sterile water and at a later stage, the concentration of extracted mRNA was determined.
To assessment the quality of mi-RNAs, the RT-PCR technique was used. The cDNA synthesis in reverse transcription reaction (RT) kit (Fermentas K1622) and 1μl oligoprimers 18 (dT) was performed. Following the PCR reaction 2μM dNTP, 1μg cDNA, Fermentas PCR buffer 1X, 0 / 75μM MgCl2, 1.25 U / μL Tag DNA at 95°C for 4 min, 95°C for 30s, annealing temperature 58°C for 30s, and72 °C for30 seconds, 35 cycles were performed. Then 1.5% agarose gel, the PCR product was dumped in wells after electrophorus is with ethidium bromide staining and color was evaluated.
Results
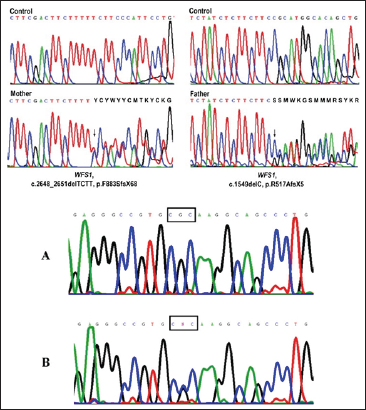
Figure 5: Schematic of the bond formation pattern in the WFS1 gene in the patient group and control group.
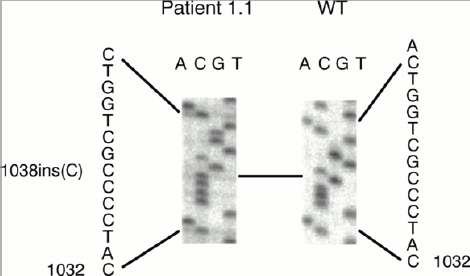
Figure 6: schematic representation of the promoter expression pattern of CISD2 gene in the patients and control group.

Figure 7: Schematic of mutations in the WFS1 gene in the patient group and control group.
Discussion and Conclusion
According to the results of sequencing the genome of patients with Wolfram Syndrome, and the genetic mutations WFS1 and CISD2genes found that about 100% of patients with Wolfram Syndrome, they have these genetic mutations. Patients with Wolfram Syndrome, unusual and frightening images in the process of Wolfram Syndrome, experience. Lot epigenetic factors involved in Wolfram Syndrome. But the most prominent factor to induce Wolfram Syndrome, mutations is WFS1 and CISD2 genes. This genes can induce the birth and can also be induced in the adulthood.
Read More About Lupine Publishers Online Journal of Neurology and Brain Disorders (OJNBD) Please Click on Below Link: https://brain-disorders-lupine-publishers.blogspot.com/

No comments:
Post a Comment
Note: only a member of this blog may post a comment.