Lupine Publishers | Journal of Immunology & Infectious Diseases
Abstract
Chediak-Higashi syndrome (SCH) is a rare autosomal recessive genetic disorder characterized by oculo-cutaneous albinism, immunodeficiency responsible for recurrent infections, predisposition to bleeding, And late neurological deterioration. The pathognomonic sign is the presence of giant intracytoplasmic granules in most of the cells of the organism but often they are identified in peripheral blood. In 85% of cases, CHS patients develop the accelerated phase characterized by an Hemophagocytic Lymphohistiocytosis syndrome (HLH) responsible for a high mortality rate. The only current effective treatment of haematological and immunological abnormalities remains allogeneic bone marrow transplantation, but without impact on skin manifestations or subsequent neurological deterioration. It is all the more effective as it is performed before the onset of an HLH syndrome.
Introduction
Chediak-Higashi syndrome (SCH) is a rare autosomal recessive genetic disorder characterized by oculo-cutaneous albinism, immunodeficiency by cytotoxic activity of T lymphocytes and natural killer cells responsible for recurrent infections, predisposition to bleeding, And late neurological deterioration. According to the International Union of Immunological Societies, the SCH is a primary immunodeficiency by immune dysregulation belonging to familial lymphohistiocytic haematophagocytosis syndromes (HFH) with hypopigmentation [1]. The LYST-CHS1 (Lysosomal Trafficking Regulator Gene) gene was identified on the long arm of chromosome 1 in 1q42-q43 [2,3]. This gene encodes the CHS protein whose exact function remains imprecise. About 500 cases have been reported [4,5]. The diagnosis is oriented by the clinical signs and facilitated by the study of the microscopic aspect of the hair which highlights the presence of pigment aggregates; But the pathognomonic sign of the disease is the presence of giant intracytoplasmic granulations in most cells of the organism [6], especially in peripheral blood or bone marrow. Approximately 85% of patients develop an acceleration phase characterized by a syndrome of lymphohocytic hemophagocytosis (HLH), which occurs during the first decade, rarely present at the onset of the disease [7]; It is fatal in the absence of treatment [8]. Currently, the only effective therapeutic option is bone marrow transplantation, which improves haematological and immune abnormalities, but does not prevent subsequent neurological deterioration. The prognosis remains poor in the absence of a bone marrow transplant, the death often occurring before the age of ten years.
Patients and Methods
They are four children followed in the pediatric department of the CHU Mustapha of Algiers for Chediak-Higashi syndrome between 2014 and 2017. The diagnosis was focused on the clinical manifestations, the presence of giant intra-leukocyte granulations. A genetic study in three children confirmed the diagnosis.
Results
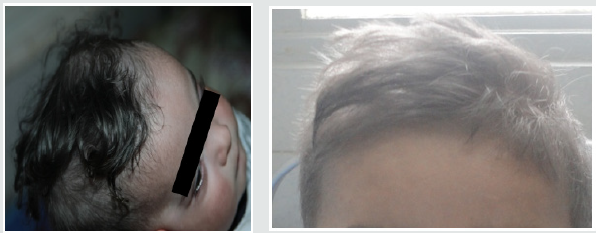
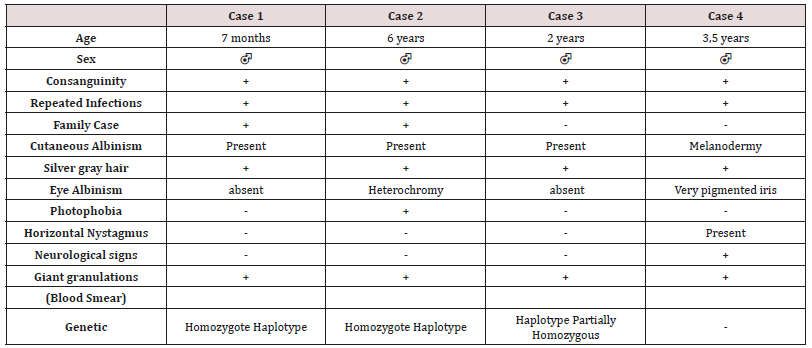
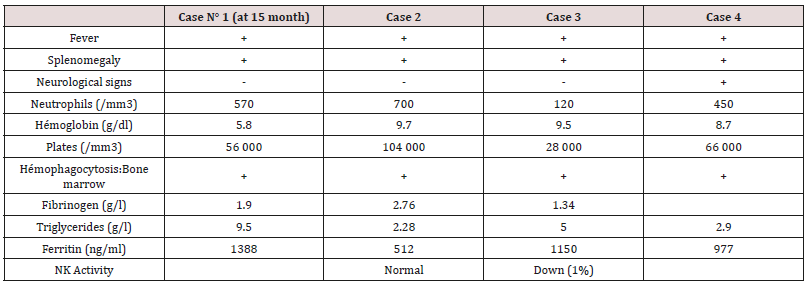
These are three boys and one daughter with an average age of diagnosis of 3.2 years (7 months - 6 years). Consanguinity is found in all cases as well as a family form (2 brothers). Three patients have a history of repeated infections. Children who were vaccinated did not report any particular incidents. Clinically, oculocutaneous albinism is present in 3 children (Figure 1) and melanoderma with a highly pigmented iris in 1 case (Table 1). Silver gray hair is present in all patient (Figure 2). The peripheral blood smear allowed to make the diagnosis by showing the intra-cytoplasmic giant granulations in all the patients. Microscopic study of the hair found deposits of melanin in irregular clods in the hair shaft in favor of Chediak-Higashi syndrome (Figure 3). A syndrome of lymphohistiocyte hemophagocytosis (HLH) (Table 2) is present in 3 cases and then in one case 8 months after the diagnosis.
Figure 1: Hypopigmentation of the skin Clinically, oculocutaneous albinism is present in 3 children hypo pigmentation of the left iris
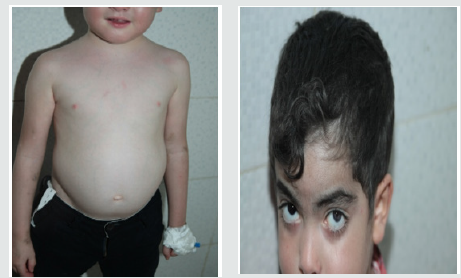
Figure 3: Deposition of melanin in the hair shaft Microscopic study of the hair found deposits of melanin in irregular clods in the hair shaft in favor of Chediak-Higashi syndrome

Melanoderma with a Highly Pigmented Iris in 1 Case All patients with signs of lymphohistiocytic activation were put on HLH 2004 protocol. The evolution is marked by a good tolerance of the treatment. One child received an allogeneic marrow transplant but died six months later. The remaining three patients are still alive and clinically stable.
Discussion
Autosomal recessive genetic disease very rare, Chediak-Higashi syndrome affects all races and all age groups. Less than 500 cases have been reported [4,5]. Most often they are reported cases or small series published. Prevalence is difficult to determine due to reported cases more than once, and other unreported cases. The largest published series (15 cases) was reported in Japan over a period of ten years [9]. Inbreeding in all our patients is reported in 50 to 85% of cases [5]. The LYST / CHS1 gene of the disease encodes the cytosolic CHS protein whose function remains imprecise. It would play a role in the exocytosis of proteins from late multivesicular endosomes. More than sixty mutations have been reported in the literature (false sense, nonsense, deletion, insertions) [10]. Mutations in the LYST / CHS1 gene lead to an abnormal function of the CHS protein with an impairment of intracytoplasmic transport, protein sequestration in giant intracytoplasmic structures and blocking of the secretory function, in particular that of leucocytes and melanocytes. Phenotype / genotype correlations were reported, thus a deletion-type mutation correlated with the early and fulminant onset of the acceleration phase, while a missense-like mutation correlated with a better prognosis with no acceleration phase and neurological deterioration [11]. Clinical signs begin after birth, or before the age of 5 years. Oculocutaneous albinism (AOC) is an important sign of diagnostic orientation, present in three of our patients, characterized by hypopigmentation which generally affects the skin, the hair and the eyes. It is related to pathological aggregation and an unequal distribution of melanosomes. The AOC may be present from birth, and concern the three organs or some of them, total or partial or even absent [12]. Sometimes hyperpigmentation as in one of our patients can exceptionally be seen, delaying the diagnosis [13,14]. Most patients have photosensitivity. Some patients have an atypical phenotype with an attenuated form, or the AOC is subtle or absent and probably unknown [11,15]. Rarely other skin lesions are observed as hyperhydrosis, erythema multiforme. The hypopigmentation of the hair gives them a blonde, gray or white color, often with a silvery or metallic luster. The eyes are blue in color and hypo pigmentation of the iris may be associated with decreased pigmentation of the retina, and ocular manifestations such as photophobia, decreased visual acuity, nystagmus, and strabismus Frequent and recurrent infections are common in childhood. Often severe, they are related to a defect in T cell cytotoxicity, NK function, and a decrease in the chemotactic and bactericidal activity of the granulocytes [16,17]. Pyogenic infections are the most frequent, especially in the skin, upper airways and mucous membranes. The most frequently isolated germs are staphylococcus aureus, β-hemolytic streptococci and pneumococcus. The involvement of the oral cavity manifests in the form of gingivitis, gingival haemorrhage, early falls of the teeth. Mouth ulcers have been described. Periodontitis has been identified as a manifestation of immune dysfunction. The bleeding tendency in these patients is related to a deficiency in the storage pool of dense granules and a defect in platelet aggregation. Haemorrhagic manifestations are usually benign and usually do not require treatment. The accelerated phase of the disease is the most important and dangerous complication of the SCH. It is responsible for a high rate of mortality within a few months [18]. It can occur at any age but especially during the first decade (85%) [19]. Our patients developed this accelerated phase before the age of 6 years. Rarely, it is the first manifestation [7,20]. Its early onset is associated with the existence of a deletion-like genetic mutation [11] and has a collapsed or absent activity in cytotoxic LTs [21]. It is manifested by a syndrome of lymphohystiocytic haematophagocytosis (HLH) whose factors triggering this acceleration are not clear. The role of EBV infection found in one of our patients was raised without this relationship being established [22]. The diagnosis of accelerated phase disease is based on the criteria of the Histiocytic Society 2004 [23]. Neurological manifestations occur in about 50% of cases and may occur at any time in childhood or adulthood, they are variable: peripheral neuropathy, coma, convulsions, ataxia, cognitive disorders, impaired balance, movement abnormalities and mental alterations. The bone marrow transplant does not prevent their subsequent appearance [24]. The diagnosis of SCH should be early, often done at around 6 years of age, but in about 25% of cases the diagnosis is delayed after age 10 [12] for our patients. The average age at diagnosis was 3.2 years. It is suspected on the clinical elements, facilitated by the microscopic study of the hair which shows aggregates of melanin pigments found in all our patients, this aspect allows the differential diagnosis with other types of cutaneous hypopigmentation. But the pathognomonic sign of the disease is the presence of giant intracytoplasmic granules in most of the cells of the organism [6] but often they are identified in peripheral blood as was the case of our patients or in the bone marrow. The diagnosis is confirmed by a genetic test for the mutation LYST. Antenatal diagnosis of the disease is possible in the cells of the chorionic villi, amniotic fluid, leucocytes of the fetal cord [25]. As with Chediak-Higashi disease, other genetic immune deficiencies are accompanied by partial oculocutaneous albinism, such as Griscelli’s disease and Hermansky-Pudlak syndrome. The distinction can be made unambiguously by the different appearance of pigment clusters in the hair sheath, much finer in the case of CHS, and especially by the presence of giant intracytoplasmic granules observed only in the CHS. However, in some cases of myeloid leukemia, one can see giant granulations called pseudo Chediak- Higashi anomaly [26]. The treatment of Chediak-Higashi disease is multidisciplinary and is based on the management of complications of the disease, treatment of the “accelerated phase” or HLH and especially the transplantation of hematopoietic stem cells. Symptomatic treatment of Chediak-Higashi disease is based on effective antibiotic therapy against infections and transfusions of blood derivatives to fight anemia and hemorrhagic complications. Eye disorders should be corrected. The eyes and skin should be protected from UV rays. Vaccinations are generally well tolerated as was the case for our patients and must be done. Hygiene and oral health care are Primordial. The occurrence of neurological symptoms and their progression must be dealt with early enough by a rehabilitation specialist. In the case of an acceleration phase (HLH, a treatment combining corticosteroids, VP16, cyclosporin and intrathecal injections of MTX (HLH 2004) [23] is introduced to achieve remission, which occurs in 75% of cases [27], but relapses are frequent and response to treatment decreases over time. When transplantation is achieved, transplantation is recommended. In SCH patients with HLH by EBV the addition of Rituximab could improve treatment [28]. In the case of refractory HLH, another therapeutic option including a monoclonal anti-CD52 antibody (Alemtuzumab) [29] is possible as a second line treatment before bone marrow transplantation. Allogeneic bone marrow transplantation (BMT) is the only current effective treatment that heals hematologic and immunological abnormalities, but has no effect on oculocutaneous albinism or subsequent neurological deterioration [9,24,30]. The pre-graft conditioning regimen comprises a combination of etoposide, busulfan, cyclophosphamide [31]. Reduced intensity of pre-graft conditioning with fludarabine, melphalan, and alemtuzumab resulted in increased survival in primary or family HLH with lower toxicity [32,33]. Bone marrow transplantation is most effective when performed before the accelerated phase occurs [31]. Patients with a profound decrease in the cytotoxic function of T lymphocytes (CTL) have a high risk of developing lymphocytic syndrome (HLH), so their screening may be an indication for early marrow transplantation [21]. The overall survival rate after marrow transplantation is 60-70% (30-32) The prognosis remains poor in the absence of a bone marrow transplant, death occurs frequently during the first decade by infections or development of an accelerated phase HLH [34] About 10 percent of patients who survive in early childhood will develop severe neurological disorders in adolescence and early adulthood [35].
Conclusion
Chediak-Higashi syndrome is a rare disease, the diagnosis is suspected in a child with oculocutaneous albinism with recurrent infections. The majority of clinical forms are early “infantile” lethal in the absence of treatment. A minority of patients present with an “attenuated” form of the disease will survive after childhood but develop an associated neuro-degerative disease. In all cases early diagnosis should be posed by a simple examination, peripheral blood smear which shows the presence of giant intracytoplasmic granules pathognomonic of this condition. The only current effective treatment of haematological and immunological abnormalities remains allogeneic bone marrow transplantation, but without impact on skin manifestations or subsequent neurological deterioration. It is more effective when it is performed before the onset of an HLH syndrome. In the event of the occurrence of accelerated phase (HLH), a treatment according to the HLH 2004 protocol is instituted in order to obtain a remission before the bone marrow transplant. The prognosis of infant form is poor, with death occurring frequently in the first decade of life through infections or development of HLH. The search for predictive factors for the development of HLH may help in the indication of early bone marrow transplantation.
Read More About Lupine Publishers Journal of Immunology Please Click on Below Link:
https://lupine-immunology-infectious-disease.blogspot.com/

No comments:
Post a Comment
Note: only a member of this blog may post a comment.