Lupine Publishers| Journal of Textile and Fashion Designing
Abstract
Dyeing in ancient times was conducted from natural animal and vegetable resources till artificial dyes were discovered in the middle of the 19th century in coal tar. Mordating using inorganic compounds to fix the dyes on textile material was responsible for the creation of alumina for the growing aluminum industry at the end of that century. Now inorganic material like asbestos can be dyed with synthetic dyes to render it less toxic and some minerals are dyed to enhance their beauty.
Natural and Synthetic Dyes
Kermes is an insect found on the oak kermes was collected by the ancient Egyptians, killed by exposure to vapours of vinegar, and dried. The product was then used as a scarlet dye with alum as a mordant. Kermes is from Arabic "qirmis" meaning deep red. Fifteen hundred years before Christ, the people of Tyre in present day Lebanon produced the famous Tyrian purple from shell fish. Indigo has been known in India and Egypt from remote periods of antiquity as indico. It began to be imported to Europe in 1516 by the way of Cape of Good Hope. Around 1587, the monopoly of cochineal dye industry (red dye from the bodies of cochineal bugs of Central America) was controlled by Spain.
The blue dye was obtained by steeping the plant in water to allow fermentation followed by the oxidation in air of the obtained solution. The fermentation is due to enzymes present in the plant which cause hydrolysis of the glucoside and liberation of the precursors of indigo blue. The structure of indigo was elucidated by Adolf von Baeyer (1835-1917) in 1880 and the synthetic product put on the market in 1897 by the Badische Anilin- und Soda Fabrik in Ludwigshafen.
The leaves of a shrub known to-day in Egypt as hennah were used by the ancient Egyptians, much as they are to-day, in the form of a paste to colour red the palms of the hands, the soles of the feet, the nails, and hair. The plant is also known as madder and was used in India. About the time of the Crusades the cultivation of madder was introduced into Italy and France. The roots were removed from the ground, washed, dried, and then finely ground. The colouring matter alizarin was isolated by European chemists at the beginning of the nineteenth century, its structure elucidated, and in 1868 synthesized by Carl Graebe (1841-1927) and Carl Theodor Liebermann (1842-1914), and immediately manufactured on large scale [1-6].
Figure 1: William Henry Perkin (1838-1907).

With the discovery of Brazil, a new market for the so-called "brazilwood" came into existence-a bright red wood that became popular for cabinet work but also for the extraction of a red dye. The logs were rasped to a coarse powder, moistened with water and allowed to ferment for weeks. The water extract gave bright red colour with fabrics mordanted with aluminum or tin salts. The colouring principle of brazilwood was isolated by the French chemist Michel Eugene Chevreul (1786-1889), who called it brazilin. In 1856, William Henry Perkin (1838-1907) (Figure 1), while experimenting with coal tar in the hope of finding artificial quinine as a cure for malaria, discovered the first violet synthetic dyestuff which he called Mauve. Since then the synthetic dye industry flourished.
Mordanting and the Aluminum Industry
The Bayer process used today for the production of alumina for the growing aluminum industry was originally discovered in 1888 in Saint Petersburg in Russia in the Tentelev Chemical Plant for supplying mordants to the textile industry. Karl Josef Bayer (1847-1904) prepared aluminum hydroxide by seeding a solution of sodium aluminate obtained by sintering bauxite with sodium carbonate. In 1889 he eliminated the sintering process and used an autoclave to obtain sodium aluminate. The modified process is used universally for the treating of bauxite.
Dyeing of Minerals
Figure 2: Howlite before and after dyeing.
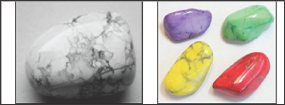
Minerals can be dyed with organic dyes. It takes few minutes at room temperature to dye a porous and a large surface area mineral like asbestos while it takes few weeks to dye a nonporous mineral like quartz, In some cases it is necessary to heat the mineral then quench it in the dye solution so that the dye can penetrate in the microscopic cracks formed along cleavage planes. Dye often improves the appearance of low-quality pearls. The process has been used for turquoise, lapis lazuli, howlite, nephrite jade, chalcedony, quartz, emerald, and ruby. For example, howlite is a calcium borosilicate hydroxide, Ca2B5SiO9(OH)5, discovered in 1868 by Henry How (1828-1879), a Canadian mineralogist. Because of its porous texture, howlite can be easily dyed (Figure 2). Heating can remove unwanted inclusions in some amethysts which make it look opaque. Heating can intensify, or even induce, a blue coloration in sapphires. Heating yellowish pink topaz sometimes has the effect of removing the yellowish color component, thereby intensifying the pink color. Most citrine is made by heating amethyst.
Asbestos, a hydrated magnesium silicate Mg3(Si2O5)(OH)4, is banned today because the fibers proved to be toxic. Research work conducted at Laval University in the 1990's proved that dyeing the fibers in aqueous solutions with certain organic dyes like Thiazol Yellow. The dye forms a chelate with the magnesium ion in the fiber that renders it nontoxic. Unfortunately, it was too late to save the industry. On the other hand, dyes are used to enhance the beauty of certain semiprecious stones.
Read More About Lupine Publishers Journal of Textile and Fashion Designing Please Click on Below Link: https://fashion-technology-lupine-publishers.blogspot.com/

No comments:
Post a Comment
Note: only a member of this blog may post a comment.