Lupine Publishers | Journal of Oncology
Abbrevations: AML: Acute Myeloidleukaemia; CBF: Core Binding Factor; mAb: Monoclonal Antibody; MDS: Myelodysplastic Syndrome
Editorial
The immune suppressive mechanisms displayed by malignant cells are considered a central process in the pathogenesis of cancer. Research in this area has gained significant momentu mover the past 20 years, with several immune checkpoints identified, including; CTLA-4, CD200/CD200R, Tim-3/Galectin-9 and PD-L1/PD-1 (Figure 1). Whilst characterising the molecular basis of leukaemia for risk stratification remains at the forefront of AML research; this must now extend to understating how the seimmune checkpoint path ways fit into the equation. A good example of why this is important is to consider CD200expression level in AML, which is a negative prognostic indicator [1]. CD200 is an immunosuppressive lig and, that when engaged with its receptor CD200R, has the capacity to attenuate T-cell and NK-cell anti-tumour activity in AML. Interestingly, most cases of CBF AML express high levels of CD200, yet CBF AML performs relatively well clinically. This paradox suggests there is a complex interplay between AML molecular heterogeneity and immune surveillance. Given the recent development and FDA approval of several immune checkpoint therapies, a full understanding of these processes and integration with standard molecular risk stratification is warranted.
Figure 1: Illustrated are immune checkpoint legends expressed on AML blast cells (left) with the cognate T-cell receptors (right), including; CD200/CD200R, PD-L1/PD-1, CTLA-4, CD47 and Galectin-9/Tim-3. Currently clinical trials are exploring the therapeutic potential CD200, PD-1 and CTLA-4 with the mAb’s Samalizumab, Pembrolizumab/Nivolumab and Ipilimumab respectively. CD47 mAb therapy is at a preclinical stage.
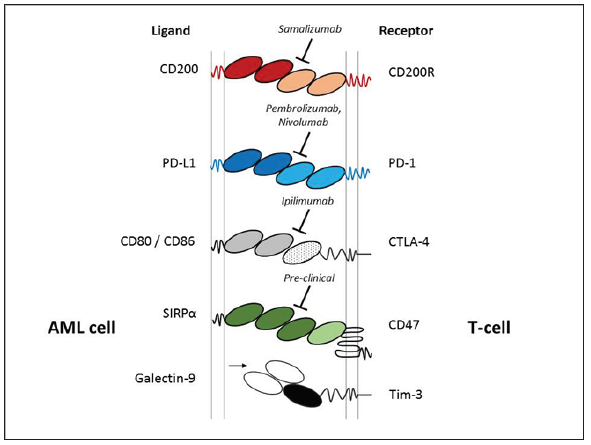
The immune checkpoint story is becoming complex for AML, since several studies report that that these immune surveillance pathways function in tandem. For example, the Galectin-9/Tim-3 immune checkpoint has been shown to cooperate with the PDL1/ PD-1 pathway in AML, which is central in driving CD8+ T-cell exhaustion. Thus targeting both Tim-3/Galectin-9 and PD-L1/PD-1 was required to achieve significant cyto reduction and improved survival in pre-clinical models [2]. Another study illustrated that the CD200/CD200R and PD-L1/PD-1 immune checkpoints are also linked in AML. In this instance, activation of CD200R was sufficient to drive the up regulation of PD-1 on memory CD8 T-cells. Further analysis relaveled that targeting both CD200/CD200R and PD-L1/ PD-1 immune checkpoints were required to significantly restore memory CD8 T-cell function [3].
This finding indicates that these immune checkpoints may be important in driving AML relapse. Indeed, this notion is realised in a current phase-II trial (NCT02708641), which is assessing the effects of the PD-L1/PD-1 checkpoint inhibitor ‘Pembrolizumab’ as a post-remission treatment in AML. The potential use of targeting the immune checkpoint in post-remission is also recognised in findings published from a recent phase-IB/II study involving the PD-L1/PD-1 checkpoint inhibitor ‘Nivolumab’. The report shows that when used in combination with azacytidine for relapsed AML, Nivolumab showed an improvement in prognosis and increased numbers of effectors CD8+ T-cells [4]. Given that the PD-L1/ PD-1 checkpoint functions in combination with other immune checkpoints, the question now is to understand whether targeting a combination of these pathways in AML performs well clinically.
To this end, results from a current phase-II trial (NCT02530463) targeting PD-L11/PD-1 with Nivolumab in combination with the CTLA-4 inhibitor ‘Ipilimumab’ in MDS are eagerly awaited. As are the next generation of therapeutic mAb’s such as ‘Samalizumab’ (Alexion Pharmaceuticals), which is designed to target the CD200/ CD200R checkpoint. The potential for immune checkpoint therapy in AML is clearly evident, however the interplay between these pathways needs full appreciation and placed into context with molecular stratification and standard therapy (Figure 1).
Read More About Lupine Publishers Journal of Oncology Please Click on below Link:
https://lupine-publishers-cancer-journal.blogspot.com/
No comments:
Post a Comment
Note: only a member of this blog may post a comment.