Lupine Publishers | Journal of Organic and Inorganic Chemical Sciences
Abstract
In the presented investigation, some novel tri-heterocyclic benzamides, 8a-g, were synthesized in several steps. First, the electrophilic benzamide, {4-[3-(chloromethyl)benzoyl]-1-piperazinyl}(2-furyl) methanone (3), was synthesized by the reaction of 2- Furoyl-1-piperazine (1) and 3-chloromethylbenzoyl chloride (2). In second series of steps, different carboxylic acids, 4a-g, were refluxed with ethanol and conc. sulfuric acid to form esters, 5a-g. These esters were further refluxed with N2H4.H2O in methanol solution to acquire acid respective hydrazides, 6a-g. These hydrazides were cyclized by refluxing with KOH, ethanol and CS2 into corresponding 1,3,4-oxadiazoles, 7a-g. In the final step, the electrophile, 3, was coupled with synthesized 1,3,4-oxadiazoles, 7a-g, in acetonitrile and potassium carbonate to acquire the targeted tri-heterocyclic molecules, 8a-g. The structural characterization of these novel compounds 8a-g was done by IR, 1H-NMR, 13C-NMR and EI-MS spectral data. These synthesized molecules were subjected to antibacterial and enzyme inhibitory and cytotoxicity evaluation. Among the series, 8b exhibit good enzyme inhibition whereas 8c exhibited good antibacterial and antifungal potential against B. subtilis, E. coli and A. flavus strains, respectively. Most of the molecules possessed moderate cytotoxicity and hence these can be utilized as possible therapeutic agents.
Keywords: Tri-Heterocyclic; 1H-NMR; 13C-NMR; EI-MS; Hemolytic Activity; Enzyme Inhibition; Antibacterial; Antifungal activity
Introduction
Ox diazole is a heterocyclic five membered ring bearing two nitrogen atoms and one oxygen atom. It has four different isomers among these four different isomers, 1,3,4-oxadiazole has been synthesized in the presented work and subjected to formation of some new derivatives [1]. A lot of derivatives of 1,3,4-oxadiazole has been synthesized by substituting at second and fifth positions of the ring. They undergo a variety of organic reactions such as electrophonic substitution, nucleophilic substitution, thermal and photochemical reactions and hence act as a medicinal backbone on which a number of valuable molecules have been constructed [2] . A broad spectrum of biological activities reported for different substituted 1,3,4-oxadiazole derivatives include anticonvulsive [3]. Muscle relaxant [4]. Herbicidal, antimicrobial [5]. Insecticidal [6]. Antibacterial, anti-inflammatory [7,8]. Analgesic, hypoglycemic [9,10]. Anti-Parkinson, anti-mitotic, anticancer [11]. Anti-tubercular [12]. Tranquilizing, anti-proliferative, antifungal [13]. Anti-HIV, anti-depressive etc [14]. Piperazine nucleus is one of the most important heterocycles exhibiting remarkable pharmacological activities. Piperazine is a heterocyclic compound, containing nitrogen atoms at opposite positions in six membered ring [15]. The piperazine scaffold has been classified as a privileged structure and is frequently found in biologically active compounds across a number of different therapeutic areas [16]. This motif is found in drug candidates displaying anti-depressant, analgesic, anti- allergenic, antibacterial, anti-cancer, anti-psychotic, anti-migraine, gastrointestinal agent and cardio tonic agent [17-23]. Thus, it appears that the piperazine core acts as a privileged structural element for the construction of bioactive molecules [24]. In order to find new drug candidates, the piperazine ring is encompassed in the new synthesized compounds along with 1,3,4-oxadizole. The coupling of multiple functionalities was considered to boost up the bioactivity potential of resulting molecules. The presented work covers synthesis of new compounds having different bioactive moieties like piperazine, 1,3,4-oxadiazole and carbamate. The antibacterial, enzyme inhibition and hemolytic potential have also been demonstrated for all the synthesized molecules. The present work was aimed to synthesize new molecules in order to evaluate their bioactivity potential against certain bacterial strains and enzymes.
Experimental
Chemistry
Purity was checked on thin layer chromatography (TLC) on pre-coated silica gel G-25-UV254 plates using different percentage of ethyl acetate and n-hexane giving single spot. Griffin and George melting point apparatus was used to record the melting points of the synthesized compounds by open capillary tube and were uncorrected. Jasco-320-A spectrophotometer was used for the IR spectra (wave number in cm-1). 1H-NMR spectra were recorded in CDCl3 on a Bruker spectrometers operating at 600 MHz. 13C-NMR spectra were recorded in CDCl3 on a Bruker spectrometers operating at 150MHz. Chemical shifts were recorded in ppm. Mass spectra (EI-MS) were taken on a JMS-HX-110 spectrometer, with complete data system.
General Synthesis of {4-[3-(chloromethyl)benzoyl]-1- Piperazinyl}(2-furyl)Methanone (3)
1-(2-Furoyl)piperazine (12.8mmol; 1) was taken in an iodine flask (250mL) containing 15.0mL of distilled water and 10 % Na2CO3 solution to adjust pH at 9-10. Then equimolar 3-chloromethylbenzoyl chloride (2) was added drop wise to the reaction medium in 2-5min. After complete addition, the iodine flask was vigorously shaken (manually) and then set to stir at room temperature for 4 hours till the formation of solid precipitates. The progress of reaction was monitored by thin layer chromatography (TLC) till single spot. The obtained precipitates were filtered, washed with distilled water and dried to yield the titled electrophile, 3.
Procedure for the synthesis of ethyl carboxylates (5a-g)
Carboxylic acids (50mmol; 4a-g) were refluxed for 3-4 hrs with 40mL EtOH in a 250mL round bottom flask in the presence of conc. H2SO4 (0.5mL/g acid). After maximal completion by TLC, excess water was added and pH was adjusted to 8-10 by aq. Na2CO3 soln (10%). The title compounds, 5a-g, were extracted by chloroform.
Procedure for the synthesis of carbohydrazides (6a-g)
Compounds 5a-g (40mmol) and N2H4.H2O (40mmol) were refluxed for 4-6 hrs in 25mL MeOH in a 100mL RB flask. After final TLC, excess water was added to acquire precipitates, 6a-g, which was separated by filtration.
General procedure for the synthesis of 5-substituted- 1,3,4-oxadiazol-2-thiols (7a-g)
Compounds 6a-g (30mmol) were refluxed for 0.5 hr with solid KOH (30mmol) in 45mL EtOH in a 100mL RB flask. Then CS2 (60mmol) was added and further refluxed for 3-6 hrs. After final TLC, excess water was added followed by conc. HCl to adjust pH of 2. The mixture was left for 3 hrs for precipitation. Precipitates, 7a-g, were collected through filtration and washed with water
General synthesis of tri-heterocyclic molecules (8a-g)
The respective 5-substituted-1,3,4-oxadiazol-2-thiol (24mmol; 7a-g, one in each reaction) was dissolved in acetonitrile (20-30mL) in 100mL round bottom flask. Then solid K2CO3 (12mmol) was added. The mixture was refluxed for half an hour and then the equimolar (24mmol) the electrophile, 3, was added. The mixture was further refluxed for 4-5 hours. Thin layer chromatography was carried out to check the reaction completion. Distilled water was added to the reaction mixture to acquire the precipitates. Precipitates were filtered, washed and dried to get the titled compounds, 8a-g.
4-[3-({[5-(2-Chlorophenyl)-1,3,4-oxadiazol-2-yl] sulfanyl}methyl)benzoyl]-1-piperazinyl}(2-furyl) methanone (8a).
Light brown liquid; yield: 84%; Molecular formula: C25H21ClN4O4S; molecular mass: 508 g/mol; IR (KBr, υmax cm-1): 3411 (N-H), 3073 (Ar C-H), 2880 (R C-H), 1654 (C=O), 1582 (Ar C=C), 1203 (C-O-C), 1106 (C-N-C), 658 (C-S); 1H-NMR (600 MHz, CDCl3, δ in ppm): 5 7.96 (d, J = 1.6 Hz, 1H, H-2"), 7.57 (dist. d, J = 1.5 Hz, 1H, H-5), 7.52 (d, J = 8.7 Hz, 1H, H-6”), 7.49-7.46* (m, 4H, H-3””, H-4””, H-5”" & H-6"”), 7.42 (t, J = 7.5 Hz, 1H, H-5"), 7.37 (dt, J = 1.5, 6.1 Hz, 1H, H-4"), 7.07 (d, J = 2.5 Hz, 1H, H-3), 6.50 (dd, J = 1.6, 3.3 Hz, 1H, H-4), 4.51 (s, 2H, H-8"), 3.84 (br s, 4H, H-3' & H-5'), 3.50 (br s, 4H, H-2' & H-6'); 13C-NMR (150 MHz, CDCl3, δ in ppm): 170.04 (C-7''), 166.00 (C-5'''), 163.40 (C-2'''), 159.21 (C-6), 147.60 (C-2), 143.95 (C-5), 136.52 (C-3"), 135.57 (C-1”), 131.77 (C-1"”), 130.77 (C-2''''), 129.21 (C-4''''), 129.08 (C-3'''') 128.12 (C-5''''), 126.92 (C- 6’”’), 126.68* (C-4” & C-5”), 126.64 (C-2”), 123.46 (C-6"), 117.10 (C-3), 111.47 (C-4), 45.50* ( C-2’, C-3’, C-5' & C-6'), 36.21 (C-8"); EI-MS (m/z): 510 [M + 2]+, 508 [M]+, 440 [C21H16ClN4O3S•+, 411 [C20H15ClN4O2S]+, 329 [C16H9ClN2O2S]+, 262 [C12H7ClN2OS•+, 246 [C13H14N2O2•+,179 [C8H4ClN2O]•+, 179 [C9H11N2O2], 151[C8H9NO2]+, 95 [C5H3O2]+.
{4-[3-({[5-(3-Aminophenyl)-1,3,4-oxadiazol-2-yl] sulfanyl}methyl)benzoyl]-1-piperazinyl}(2-furyl) methanone (8b).
Light brown liquid; yield: 81%; Molecular formula: C25H23N5O4S; molecular mass: 489 g/mol; IR (KBr, υmax cm-1): 3413 (N-H), 3071 (Ar C-H), 2886 (R C-H), 1658 (C=O), 1580 (Ar C=C), 1203 (C-O-C), 1106 (C-N-C), 653 (C-S); 1H-NMR (600 MHz, CDCl3, δ in ppm): δ 7.91 (d, J = 7.9 Hz, 1H, H-4”’’), 7.57 (s, 1H, H-2’”’), 7.56 (d, J = 1.5 Hz, 1H, H-2”), 7.54 (d, J = 8.0 Hz, 1H, H-6), 7.47 (d, J = 7.4 Hz, 1H, H-6”), 7.43 (br s, 1H, H-5), 7.42 (t, J = 7.5 Hz, 1H, H-5”"), 7.39-7.37* (m, 2H, H-4" & H-5” ), 7.06 (d, J = 2.5 Hz, 1H, H-3), 6.48 (br s, 1H, H-4), 4.52 (s, 2H, H-8"), 3.85 (br s, 4H, H-3' & H-5' ), 3.50 (br s, 4H, H-2' & H-6'); 13C-NMR (150 MHz, CDCl3, δ in ppm): 170.08 (C-7"), 166.06 (C-5’”), 163.48 (C-2’”), 159.25 (C-6), 147.69 (C-2), 143.91 (C-5), 136.57 (C-3”), 135.50 (C-1"), 132.08 (C-3’”’), 131.70 (C-1""), 130.73 (C-2''''), 129.21 (C-4'''), 128.12 (C-5''''), 126.92 (C-6''''), 126.64 (C-2"), 126.62* (C-4” & C-5"), 123.45 (C-6"), 117.10 (C-3), 111.45(C-4), 45.51*( C-2', C-3', C-5' & C-6'), 36.21(C-8"); 489 [M]+, 421 [C21H19N5O3S]+ 392 [C20H18N5O2S]+, 310 [C16H12N3O2S]+, 246 [C13H14N2O3]+, 243 [C12H9N3OS]•+,179 [C9H11N2O2]•+, 161 [C8H7N3O]+, 151 [C8H9NO2]+, 95 [C5H302]+.
{4-[3-({[5-(3-Nitrophenyl)-1,3,4-oxadiazol-2-yl] sulfanyl}methyl)benzoyl]-1-piperazinyl}(2-furyl) methanone(8c).
Light brown liquid; yield: 86%; Molecular formula: C25H23N504S; molecular mass: 519 g/mol; IR (KBr, υmax cm-1): 3412 (N-H), 3070 (Ar C-H), 2887 (R C-H), 1653 (C=O), 1580 (Ar C=C), 1205 (C-O-C), 1107 (C-N-C), 652 (C-S 1H-NMR (600 MHz, CDCl3, δ in ppm): 5 8.35 (d, J = 2.0 Hz, 1H, H-2’”’), 8.17 (d, J = 6.9 Hz, 1H, H-6’’”), 7.83 (br s, 1H, H-2''), 7.59-7.53* (m, 1H, H-4'''' & H-5''''), 7.52 (br s, 2H, H-5), 7.48-7.43* (m, 2H, H-4’’ & H-5”), 7.07 (d, J = 3.2 Hz, 1H, H-3), 6.51 (dd, J = 1.7, 3.4 Hz, 1H, H-4), 4.55 (s, 2H, H-8”), 3.84 (br s, 4H, H-3’ & H-5' ), 3.51 (br s, 4H, H-2' & H-6'); 13C-NMR (150 MHz, CDCl3, δ in ppm): 169.96 (C-7’’), 165.18 (C-5’’’), 164.23 (C-2’’’), 159.22 (C-6), 149.53 (C-3''''), 147.61 (C-2), 143.97 (C-5), 136.20 (C-3''), 135.67 (C-1’’), 130.79 (C-6’’”), 129.25 (C-5”’’), 128.88 (C-4’’), 128.78 (C- 5’’), 128.44 (C-1’’”), 128.17 (C-2’’), 127.54 (C-6’’), 126.09 (C-4’”’), 125.49 (C-2’”’), 117.21 (C-3), 111.53 (C-4), 48.00* ( C-2', C-3', C-5’ & C-6'), 36.21 (C-8”); EI-MS (m/z): 519 [M]+, 451 [[C21H19N5O3S]•+ 422 [C20H18N5O2S]+, 340 [C16H12N3O2S]•+,, 273 [C12H9N3OS]•+, 246 [C13H14N2O3]•+ 191 [C8H5N3O3]+, 179 [C9H11N202]+, 151 [C8H7N3O]+, 95 [C5H302]+.
{4-[3-({[5-(4-Methylphenyl)-1,3,4-oxadiazol-2-yl] sulfanyl}methyl)benzoyl]-1-piperazinyl}(2-furyl) methanone (8d).
Black brown liquid; yield: 88%; Molecular formula: C26H24N404S; molecular mass: 488 g/mol; IR (KBr, υmax cm-1): 3411 (N-H), 3074 (Ar C-H), 2880 (R C-H), 1658 (C=O), 1583 (Ar C=C), 1209 (C-O-C), 1109 (C-N-C), 660 (C-S); 1H-NMR (600 MHz, CDCl3, δ in ppm): 5 7.86-7.83 (m, 1H, H-2’’), 7.55 (d, J = 8.6 Hz, 1H, H-6’’), 7.53-7.52 (m, 2H, H-2’”’ & H-6’’”), 7.49 (br s, 1H, H-5), 7.42 (t, J = 7.5 Hz, 1H, H-5’’), 7.37-7.36 (m, 1H, H-4’’), 7.33 (d, J = 6.0 Hz, 2H, H-3”’’ & H-5”’’), 7.07 (d, J = 3.4 Hz, 1H, H-3), 6.50 (dd, J = 1.8, 3.4 Hz, 1H, H-4), 4.50 (s, 2H, H-8’’), 3.84 (b s, 4H, H-3' & H-5'), 3.39 (br s, 4H, H-2' & H-6'), 2.39 (s, 3H, H-7””); 13C-NMR (150 MHz, CDCl3, δ in ppm): 170.06 (C-7’’), 166.16 (C-5’’’), 163.08 (C-2’’’), 159.22 (C-6), 147.57 (C-2), 143.97 (C-5), 142.39 (C-3’’), 136.58 (C-1’’), 136.34 (C-4’’’’), 130.77 (C- 4’’), 130.38 (C-5’’), 129.20 (C-2’’’’ & C-6’’’’), 128.10 (C-3’’’’ & -5’’’’), 127.84 (C-2’’), 126.88 (C-6’’), 120.71 (C-1’”’), 117.09 (C-3), 111.52 (C-4), 46.50* ( C-2', C-3', C-5' & C-6'), 36.21 (C-8’’), 22.58 (C-7’”’); EI-MS (m/z): 488 [M]+, 420 [C22H20N403S]•+ 391 [C21H19N503]•+, 309 [C17H13N2O2S]+, 246 [C13H14N203]+ 242 [C13H14N203]•+ 179 [C9H11N202]+, 160 [C9H11N202]+, 151 [C8H7N3O]+, 95 [C5H302]+.
{4-[3-({[5-(4-Hydroxyphenyl)-1,3,4-oxadiazol-2-yl] sulfanyl}methyl)benzoyl]-1-piperazinyl}(2-furyl) methanone (8e).
Light brown liquid; yield: 85%; Molecular formula: C25H23N504S; molecular mass: 490 g/mol; IR (KBr, υmax cm-1): 3075 (Ar C-H), 2889 (R C-H), 1654 (C=O), 1585 (Ar C=C), 1201 (C-O-C), 1109 (C-N-C), 655 (C-S); 1H-NMR (600 MHz, CDCl3, δ in ppm): 5 7.71 (d, J = 8.3 Hz, 2H, H-2’’’’ & H-6’’’’), 7.53 (br s, 1H, H-2’’), 7.52 (br s, 1H, H-5), 7.46 (d, J = 6.1 Hz, 1H, H-6’’), 7.40 (t, J = 7.6 Hz, 1H, H-5”), 7.33 (d, J = 7.4 Hz, 1H, H-4’’), 7.04 (d, J = 3.4 Hz, 1H, H-3), 6.86 (d, J = 8.4 Hz, 2H, H-3’”’ & H-5”’’), 6.47 (dd, J = 1.3, 3.0 Hz, 1H, H-4), 4.43 (s, 2H, H-8’’), 3.82 (br s, 4H, H-3’ & H-5’), 3.46 (br s, 4H, H-2’ & H-6’); 13C-NMR (150 MHz, CDCl3, δ in ppm): 170.25 (C-7’’), 166.30 (C-5’’’), 162.21 (C-2’’’), 159.33 3(C-6), 147.25 (C-2), 144.21 (C-5), 136.82 (C-3’’), 135.23 (C-1’’), 130.86 (C-4’’), 129.23 (C-5”), 128.54 (C-3”’’ & C-5’”’), 128.08 (C-6’’), 126.75 (C-1”’’), 161.05 (C-4”’’), 117.34 (C-3), 116.48 (C-2’’” & C-6’’’’), 114.25 (C-2”), 111.59 (C-4), 45.49* ( C-2', C-3', C-5’ & C-6'), 36.20 (C-8’’); EI-MS (m/z): 490 [M]+, 422 [C6H2N2]+ 393 [C6H2N2]+, , 246 [C13H14N2O3]•+ 244 [C6H2N2]•+ 179 [C6H2N2]+, 161 [C6H2N2]+, 151 [C6H2N2]+, 118 [C6H2N2]+ 95 [C5H3O2]+
{4-[3-({[5-(2,4-Dichlorophenyl)-1,3,4-oxadiazol-2- yl]sulfanyl}methyl)benzoyl]-1-piperazinyl}(2-furyl) methanone (8f).
Light brown liquid; yield: 85%; Molecular formula: C25H23N5O4S; molecular mass: 542 g/mol; IR (KBr, υmax cm-1): 3414 (N-H), 3070 (Ar C-H), 2882 (R C-H), 1659 (C=O), 1579 (Ar C=C), 1198 (C-O-C), 1113 (C-N-C), 659 (C-S); 1H-NMR (600 MHz, CDCl3, δ in ppm): 5 7.86 (d, J = 1.6 Hz, 1H, H-2’’), 7.59-7.51* (m, 2H, H-5’’’’ & H-6’’’’ ), 7.55 (dist. d, J = 1.6 Hz, 1H, H-5), 7.51 (d, J = 8.7 Hz, 1H, H-6’’), 7.45 (t, J = 7.5 Hz, 1H, H-5’’), 7.49 (s, 1H, H-3’’’’), 7.32 (dt, J = 1.5, 6.1 Hz, 1H, H-4’’), 7.09 (d, J = 2.5 Hz, 1H, H-3), 6.50 (dd, J = 1.5, 3.2 Hz, 1H, H-4), 4.50 (s, 2H, H-8’’), 3.88 (br s, 4H, H-3' & H-5'), 3.48 (br s, 4H, H-2’ & H-6’); 13C-NMR (150 MHz, CDCl3, δ in ppm): 170.03 (C-7’’), 166.05 (C-5’’’), 163.48 (C-2’’’), 159.22 (C-6), 147.65 (C-2), 143.99 (C-5), 137.89 (C-4’”’), 136.58 (C-3’’), 135.59 (C-1”), 134.90 (C-1’’”), 133.72 (C-6’”’), 131.21 (C-5”’’), 129.44 (C-2’’”), 127.65 (C- 3’”’) 126.67* (C-4” & C-5’’), 126.62 (C-2’’), 123.49 (C-6”), 117.10 (C-3), 111.43 (C-4), 48.55* ( C-2', C-3', C-5' & C-6'), 36.22 (C-8’’); EI- MS (m/z): 546 [M + 4]+, 544 [M + 2]+, 542 [M]+, 474 [C5H3O2]•+ 445 [C5H3O2]+, 363 [C5H3O2]+, 296 [C5H3O2]•+ 246 [C5H3O2]•+, 213 [C5H3O2]+, 179 [C5H3O2]+, 151 [C5H3O2]+, 118 [C6H2N2O]+, 95 [C5H3O2]+.
{4-[3-({[5-(3,5-Dinitrophenyl)-1,3,4-oxadiazol-2-yl] sulfanyl}methyl)benzoyl]-1-piperazinyl}(2-furyl) methanone (8g).
Light brown liquid; yield: 84%; Molecular formula: C25H20N6O8S; molecular mass: 564 g/mol; IR (KBr, υmax cm-1): 3417 (N-H), 3068 (Ar C-H), 2889 (R C-H), 1650 (C=O), 1584 (Ar C=C), 1206 (C-O-C), 1107 (C-N-C), 652 (C-S); 1H-NMR (600 MHz, CDCl3, δ in ppm): 5 8.07 (s, 1H, H-4’”’), 7.59 (d, J = 6.3 Hz, 2H, H-2’”’ & H-6’”’), 7.53 (s, 1H, H-2”), 7.49 (br s, 1H, H-5), 7.46 (d, J = 7.4 Hz, 1H, H-6"), 7.43 (t, J = 7.7 Hz, 1H, H-5’’), 7.34 (d, J = 7.0 Hz, 1H, H-4”), 7.07 (d, J = 4.2 Hz, 1H, H-3), 6.51 (br s, 1H, H-4), 4.48 (s, 2H, H-8”), 3.86 (br s, 4H, H-3' & H-5'), 3.66 (br s, 4H, H-2' & H-6'); 13C-NMR (150 MHz, CDCl3, δ in ppm): 170.09 (C-7”), 166.05 (C-5’’’), 163.45 (C-2’”), 159.24 (C-6), 150.08 (C-3’’’’ & C-5’’’’), 147.65 (C-2), 143.95 (C-5), 136.54 (C-3’’), 135.57 (C-1’’), 134.21 (C-4’’’’), 131.40 (C-1’’’’), 130.77 (C- 2’’’’ & C-6’’’’), 126.69* (C-4’’ & C-5’’), 126.60 (C-2’’), 123.48 (C-6’’), 117.12 (C-3), 111.41 (C-4), 45.55* ( C-2’, C-3’, C-5' & C-6'), 36.21 (C- 8’’); EI-MS (m/z): 564 [M]+, 496 [C5H3O2]+ 467 [C20H15N6O6S]+, 385 [C16H9N4O6S]+, 318 [C12H6N4O5S]•+ 246 [C5H3O2]•+ 236 [C8H4N4O5]+, 179 [C9HuN2O2]+, 151 N4, 118 [C6H2N2O]+, 95 [C5H3O2]+.
Biological Activities Assays
Acetylcholinesterase (AChE) Assay
The AChE inhibition activities were performed according to the reported method with slight modifications. Total volume of the reaction mixture was 100μL. It contained 60 μL Na2HPO4 buffer with concentration of 50 mM and pH 7.7. 10 μL test compound (0.5 mM well-1) was added, followed by the addition of 10 μL (0.005 unit well-1) enzyme. The contents were mixed and pre-read at 405 nm. Then contents were pre-incubated for 10 min at 37 °C. The reaction was initiated by the addition of 10 μL of 0.5 mM well-1 substrate (acetylthiocholine iodide), followed by the addition of 10μL DTNB (0.5mM well-1). After 15 min of incubation at 37 °C absorbance was measured at 405 nm using 96-well plate reader Synergy HT, Biotek, USA. All experiments were carried out with their respective controls in triplicate. Eserine (0.5mM well-1) was used as a positive control. The percent inhibition was calculated by the help of following equation IC50 values were calculated using EZ�Fit Enzyme kinetics software (Perrella Scientific Inc. Amherst, USA) [25].
Antibacterial and antifungal Assay
Disc diffusion method was used to find out the antimicrobial activity of the synthesized compounds. 100μL suspensions of tested microorganisms was spread on PDA medium for 106 spores/mL of fungi and on NA medium for 107 colony-forming units/mL of bacteria cells. The filter discs of 6mm diameter were saturated with compound solution and placed on the agar plates inoculated with the tested microorganisms. Filter discs without samples were employed as negative control. Rifamicin (30|ig/disk) and Fluconazole (30|ig/disk) were applied as positive reference for bacterial strains and fungal strains, respectively. Plates were placed 4 °C for 2 hours and then incubated at 37 °C for 18 hours for bacterial strains and at 28 °C for 24 hours for fungal strains. Antimicrobial activity was justified after comparison of diameter of growth inhibition zone measured in mm for organisms and the controls [26].
Hemolytic Activity
Hemolytic activity of the compound was studied by the reported method. 3 mL freshly obtained heparinized bovine blood was collected. Blood was centrifuged for 5 min at 1000 x g plasma was discarded and cells were washed three times with 5 mL of chilled (4 oC) sterile isotonic phosphate-buffer saline (PBS) at pH 7.4. Erythrocytes were maintained 108 cells per mL for each assay. Hundred μL of each compound was mixed with human (108 cells/ mL) separately. Samples were incubated for 35 min at 37 oC and agitated after 10 min. Immediately after incubation the samples were placed on ice for 5 min then centrifuged for 5 min at 1000 x g. Supernatant 100μL were taken from each tube and diluted 10 time with chilled (4 oC) PBS. Triton X-100 (0.1 % v/v) was taken as positive control and phosphate buffer saline (PBS) was taken as negative control and passed through the same process. The absorbance was noted at 576 nm using nQuant (Bioteck, USA). The % RBCs lysis for each sample was calculated [27, 28].
Results and Discussion
Chemistry
Table 1: Different substituent in scheme 1.

The presented study describes the synthesis of some novel tri-heterocyclic benzamides, 8a-g, following a facile strategy in multi-steps (Scheme 1 & Table 1). In first part, an electrophonic, {4-[3-(chloromethyl)benzoyl]-1-piperazinyl}(2-furyl)methadone (3) , was synthesized by the reaction of 2-Furoyl-1-piperazine (1) and 3-chloromethylbenzoyl chloride (2). The second part consisted a series of convergent steps, where, different carboxylic acids, 4a-g, were refluxed with ethanol and conc. sulfuric acid to form esters, 5a-g. These esters were further refluxed with N2H4.H2O in methanol solution to acquire acid respective hydrazides, 6a-g. These hydrazides were cyclized by refluxing with KOH, ethanol and CS2 into corresponding 1,3,4-oxadiazoles, 7a-g. In the final part of the synthesis, the electrophile, 3, was coupled with synthesized 1,3,4-oxadiazoles, 7a-g, in acetonitrile and potassium carbonate to acquire the targeted novel tri-heterocyclic molecules, 8a-g, in good yields. Structures of these novel compounds were confirmed by IR, 1H-NMR, 13C-NMR and EI-MS techniques. The structure of one of the compounds is discussed hereby in detail for the benefit of the readers. The molecular formula, C25H22N4O5S, of 8e was established through it EI-MS spectrum showing molecular ion peak at m/z 490 g/mol. The number of protons and in its 1H-NMR, and number of carbon resonances in its 13C-NMR spectrum also supported this assignment. The IR spectrum well supported the molecular functionalities by distinct absorption bands at 3075 (Ar C-H), 2889 (R C-H), 1654 (C=O), 1585 (Ar C=C), 1201 (C-O-C), 1109 (C-N-C) and 655 (C-S). In 1H-NMR spectrum, signals of methylbenzamide moiety appeared at 5 7.53 (br.s, 1H, H-2”), 7.46 (d, J = 6.1 Hz, 1H, H-6"), 7.40 (t, J = 7.6 Hz, 1H, H-5"), 7.33 (d, J = 7.4 Hz, 1H, H-4") and 4.43 (s, 2H, CH2-8”). The signals of protons for 4-hydroxyphenyl ring appeared at 7.71 (d, J = 8.3 Hz, 2H, H-2””, H-6””) and 6.86 (d, J = 8.4 Hz, 2H, H-3””, H-5””). Furan ring showed three peaks in aromatic region at 5 7.52 (br.s, 1H, H-5), 7.04 (d, J = 3.4 Hz, 1H, H-3) and 6.47 (dd, J = 1.3, 3.0 Hz, 1H, H-4). The eight protons of piperazine ring appeared at 5 3.82 (br.s, 4H, CH2-3’, CH2-5') and 3. (br.s, 4H, CH2-2’, CH2-6') (Figure 1a & Figure 1b). The structure was also thorough supported by its 13C-NMR spectrum (Figure 2a &Z Figure 2b). The distinct peak at m/z 194 in its EI-MS spectrum was related to 5-(4-hydroxyphenyl)-1,3,4-oxadiazol-2-thiol, the peak at m/z 119 was related to 4-hydroxyphenylcyanide moiety while the peak at m/z 95 to furoyl part of the molecule (Figure 3). So, on the basis of above cumulative evidences, the molecule 8e was named as {4-[3-({[5-(4-Hydroxyphenyl)-1,3,4-oxadiazol-2-yl]sulfanyl} methyl)benzoyl]-1-piperazinyl} (2-furyl)methanone. Similarly, the structures of all other synthesized derivatives were characterized by aforesaid pattern.
Scheme 1: Outline for the synthesis of novel tri-heterocyclic benzamides. Reagents & Conditions: (I) Aq. Na2CO3 soln./pH 9-10/ stirring at RT for 4 hrs. (II) EtOH/H2SO4/refluxing for 3-4 hrs. (III) MeOH/N2H4 • H2O/refluxing for 4-6 hrs. (IV) EtOH/CS2/ KOH/refluxing for 3-6 hrs. (V) Acetonitrile/K2CO3/refluxing for 0.5 hrs for activation of 7a-g (one in each reaction), followed by addition of 3 and finally refluxing for 4-5 hrs to obtain 8a-g.
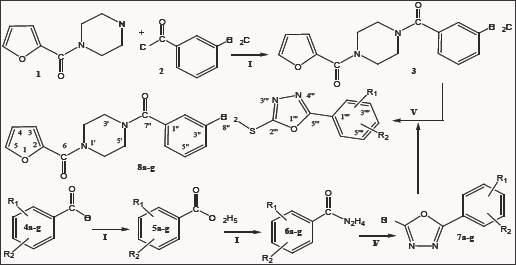
Figure 1a: Aromatic region of 1H-NMR for 8e.
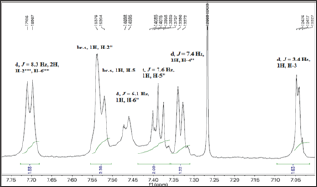
Figure 1b: Aliphatic region of 1H-NMR for 8e.
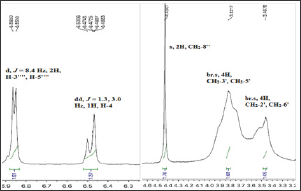
Figure 2a: Aromatic region of 13C-NMR for 8e.
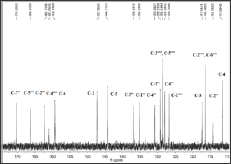
Figure 2b: Aliphatic region of 13C-NMR for 8e.
Figure 3: Mass spectrum of 8e.
Biological activities
The novel tri-heterocyclic molecules, 8a-g, were screened for biological activities such as enzyme inhibition, hemolytic activity, biological and fungal activity to ascertain their possible therapeutic potential for the associated ailments.
AChE inhibitory potential
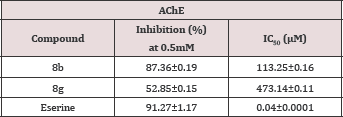
All the synthesized compounds 8a-g was screened against acetyl cholinesterase for enzyme inhibition potential. Among these synthesized compounds, the molecule 8b and 8g exhibited inhibitory potential of 87.36±0.19 and 52.85±0.15 relative to serine 91.27±1.17, a reference standard (Table 2).
Table 2: AChE inhibition of synthesized compounds, 8a-g
Antibacterial activity
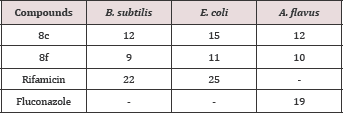
To study the antibacterial activity of the synthesized molecules, 8a-g was screened for antibacterial activity against Gram-positive stain Bacillus subtilis and Gram-negative strain Escherichia coli. Rifamicin was used as reference standard in this study (Table 3). Compounds 8c and 8f showed good inhibitory potential against the bacterial strains used in this study, especially compound 31c with values of 12 μM and 15 μM against B. subtilis and E. coli respectively.
Antifungal activity
The result of antifungal activity was also shown in Table 3. For anti-fungal activity the synthesized molecules, 8a-g were screened against A. flavus. The compounds 8c and 8f exhibited good antifungal activity against fungal strain in addition to the antibacterial activity. Fluconazole was used as reference standard in this study (Table 3).
Table 3: Antibacterial and Antifungal activities (zone of inhibition, mm) of synthesized compounds, 8a-g.
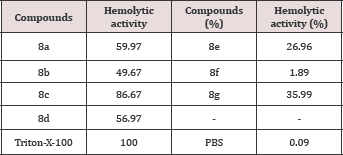
Hemolytic activity
The results of hemolytic activity assay revealed that all tested compounds ranged below the positive control Triton-X-100. Highest hemolytic activity was shown by 8c (86.67 %) which was lower than the positive control Triton-X-100 (Table 4). Other compounds mostly showed low hemolytic activity. The lowest activity was shown by 8f (1.89 %) but higher than the negative controls PBS- 0.09.
Table 4: Hemolytic activity of synthesized compounds, 8a-g.
Structure-activity relationship
Among the synthesized compounds, only two compounds remained potent against the three microbial strains taken into account. The active compounds were 8c and 8f bearing 3-nitrophenyl and 2,4-dichlorophenyl groups, respectively. These compounds showed zone of inhibition as 12, 15 & 12 and 9, 11, & 10 mm against B. subtilis, E. coli & A. flavus, respectively, with reference of 22, 25 & 19 mm. The cytotoxicity of the synthesized molecules was also investigated through hemolytic activity analysis. The highest hemolytic activity was shown by 8c (86.67 %) having bearing 3- nitrophenyl group but it was lower than the positive control (Triton-X-100). The lowest activity in the series was exhibited by 8f (1.89 %) which was incorporating 2,4-dichlorophenyl moiety in its structure. Only two compounds, 8b and 8g, bearing 3-aminophenyl and 3,5-dinitrophenyl group, respectively, remained moderately low active against AChE enzyme.
Conclusion
The structures of the synthesized novel tri-heterocyclic molecules, 8a-g, were thoroughly corroborated by spectroscopic analysis. The newly synthesized compounds were screened for enzyme inhibition, antibacterial, antifungal and hemolytic activity. The data in the (Table 1) indicated that among the synthesized compound 8b exhibit good enzyme inhibition. Some of the compounds exhibited suitable antibacterial and antifungal potential against B. subtilis, E. coli and A. flavus strains. Particularly, 8c displayed the maximum inhibition. The cytotoxic results have also been processed to evaluate the cytotoxicity of the synthesized molecules and found a few of them toxic up to some extent and others with less toxicity. From the results of various biological activities it was concluded that these compounds would be of better use in drug development to combat bacterial infections and as antifungal agents in the future.
Read More About Lupine Publishers Journal of Organic and Inorganic Chemical Sciences Please Click on Below Link:
https://lupinepublishers-chemicalsciences.blogspot.com/

No comments:
Post a Comment
Note: only a member of this blog may post a comment.