Lupine Publishers| Journal of Gastroenterology and Hepatology
Abstract
Nonalcoholic fatty liver disease (NAFLD) is described as exposition of multiplex liver metabolic disturbance interconnected with obesity. NAFLD is depicted by steatosis, excessive accumulation of fats in liver, due to triglycerides export and oxidation of fatty acid from plasma and de novo synthesis. Hepatic steatosis can therefore be explained as biochemical outcome of inconsistency between interfused mechanisms of lipid biotransformation. This condition is allied to a range of various modifications in lipoproteins, fatty acids, and glucose metabolisms in organism. So, above metabolic disfunctions are suspected to be the origin of possibility for adverse cardiometabolic risk agents related to NAFLD, like dyslipidemia, Type 2 diabetes mellitus (T2DM), and insulin resistance. Reactive oxygen species (ROS) generation participates as known inducer of inflammation and oxidative stress, that exacerbate this disease. These disorders are hallmarks that worsen NAFLD complications, so far participate in developing advanced stages of NAFLD and incline the body to CVD and T2D. The reciprocal risks exist among these diseases. Given the sharp growing prevalence and persistence of NAFLD, and its complexity that provoke additional metabolic syndrome, this review discusses various mechanisms of developing NAFLD, interaction with other associated hallmarks, aiming to clarify beneficial mechanisms for improvement.
Keywords: Nonalcoholic fatty liver disease; oxidative stress; Type 2 diabetes; noncoding RNAs; cardiovascular disease
Introduction
The liver is known as metabolically complex organ due to its
multiple biological activities, involving detoxification of various
endogenous metabolites and exogenous toxic substances, formation
of biochemicals needed for degradation and the synthesis of
protein. The liver also serves an exigent task in metabolisms like
lipid homeostasis and glycogen storage regulation [1]. Increased
activity of mitochondria consequent to the fatty acids hyper-afflux
from oxidation of fatty acid, produce free radicals that generate
liver oxidative stress [2]. Abnormal function of liver may engender
several metabolic impairments, such as NAFLD (Marrero et al.,
2002). NAFLD is explained as the grouping of excess fat into liver
cells, that does not result from alcohol consumption. The existence
of fats in the liver is normal; however, if the concentration of fats
exceeds 5%-10% of the weight of liver, then it is called a fatty liver
(steatosis). Thus, NAFLD is explained as the amassing of liver fat
of >5% of liver weight with <10g of daily alcohol consumption
(Bayne, 2010). Besides these, generation of NAFLD was discovered
to be linked with insulin resistance, the latter is known also as a
critical risk factor for developing type 2 diabetes (T2D) [3]. NAFLD
comprise a large spectrum of manifestations extending from simple
steatosis, continuing to nonalcoholic steatohepatitis (NASH) and
cirrhosis. Furthest, NAFLD correlates with significant higher risk
of developing hepatocellular carcinoma (HCC) [4]. The worse
hallmark of this disease is the significant interdependence with
various attributes of metabolic syndrome (MetS), such (T2DM),
obesity or dyslipidemia [5]. MetS is hallmarked by the aggregation
of several impairments involving elevated blood pressure, obesity,
dyslipidemia, insulin resistance and proinflammatory activities
[6]. Moreover, studies by different researchers elucidated NAFLD
as the leading inducer of liver diseases and the main reason for
impaired liver function worldwide; also considered as an inherent part of MetS (hypertension, hyperglycemia, central obesity and
dyslipidemia) [7,8]. The evolution of MetS has been coincided with
a growth in liver disorders including NAFLD, and was revealed
to correspond with disorders, like cardiovascular disease (CVD).
Particularly, NAFLD has been taken as hepatic exposition of MetS
[9].
The NAFLD prevalence increases quickly around the world
and noted as a camouflaged epidemic. The Universal estimation
of NAFLD prevalence in general population has overpassed 25%
[10], See Figure 1. In developing countries such as, China; and
India, the number of NAFLD patients has been augmenting sharply
over the last decades [11]. However, no medications presently
approved for NAFLD. The primary therapeutic intervention in
NAFLD, same as in other MetS is gained from lifestyle improvement,
promoting equitable low-energy diet, together with promoting
physical activity. So, these are prime remunerative approaches
for this condition [12,13]. Lifestyle moderation has improved
the metabolism syndrome features; however, more effort needs
to be made in addressing various MetS components [14]. Thus,
to provide better understanding that will help different players
involved in management of MetS disorders. This review discusses
various mechanisms of developing NAFLD and its interplay with
other metabolic disorders, while clarifying beneficial mechanisms
for improvement.
Figure 1: NAFLD Prevalence in global remarkable regions.
N. America; North America, S. America; South America, Euro; Europe, M. East; Middle East.
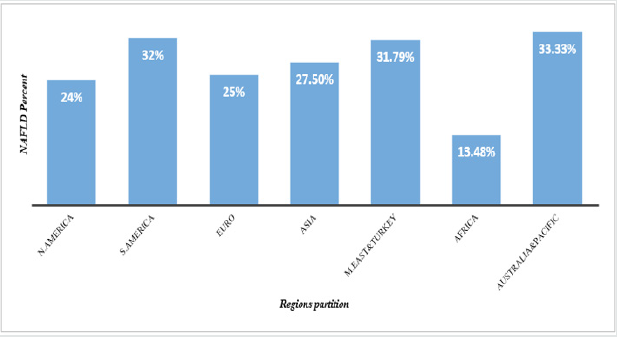
Prevalence of NAFLD
The NAFLD prevalence increases quickly in every region in the
world as a masked epidemic. The worldly estimation of NAFLD
prevalence in general population has surpassed 25% [10] (Figure
2).
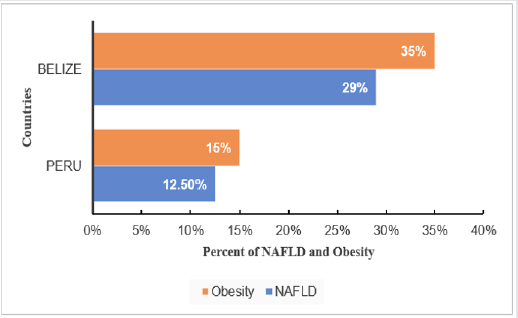
In region of South and Central America, NAFLD prevalence
varies depending on obesity rate, where Belize has the highest
obesity prevalence of 35% with NAFLD 29% and Peru has the
lowest obesity prevalence of 15% with NAFLD 12.5% [10]. The Occurrence of obesity aggravate NAFLD prevalence. In addition,
NAFLD is sharply elevating worldwide, coincident with the
augmented prevalence of obesity. Currently NAFLD has become the
major chronic liver disease; NAFLD prevalence in adult population
of developed countries is approximately 30% [9]. NAFLD has also
become a considerable liver disease in children in response to the
elevation of childhood obesity prevalence. Obesity may initiate
production of excess ROS and systemic oxidative stress [15] which
result in protein and lipid oxidation [16] (Figure 3).
Figure 3: Influence of Genetic PNPLA3 on NAFLD in USA.
H. Puerto Rican; Hispanic from Puerto Rican origin, H. Dominican; Hispanic from Dominican origin, H. Mexican; Hispanic
from Mexican origin.
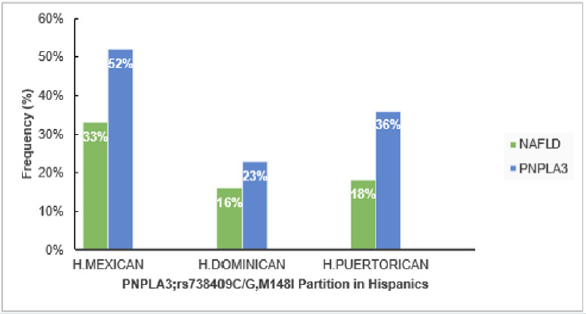
The study from sub-region of USA that compared NAFLD in Hispanic from different origins, showed the greatest NAFLD prevalence in Hispanics from Mexican origin (33%) than those from Caribbean origin (Puerto Rica (18%), Dominic Republic (16%)), (P<0.01). The greater NAFLD prevalence remained in Hispanics of Mexicans than Dominican or Puerto Rican origin, even after regulating other factors that discovered to contribute in NAFLD; such as insulin resistance, levels of triglyceride and C-reactive protein, hypertension, serum level of high-density lipoprotein, waist circumference, body mass index (BMI), sex and age [17]. Prevalence of NAFLD might be explained by elevated polymorphism prevalence in the gene encoding patatin-like phospholipase domain-containing 3 (PNPLA3; rs738409 C/G, M148I); especially in Hispanics where it accounts (49%), compared to Non-Hispanics whites (23%) and African-American (17%) [18,19]. Moreover, PNPLA3; rs738409 counts 52% in Hispanics Mexican, 23% in Dominicans [20] (Eric et al., 2019) and 36% in Hispanics Puerto Rican [21]. According to a genome-wide association study [19] elucidated that a non-synonymous sequence variation (rs738409) in PNPLA3 that replaces methionine for isoleucine at residue 148 (I148M) correlates with disproportion in liver lipid profile and the possibility of generating NAFLD. Thus, higher PNPLA3; rs738409 C/G, M148I in Hispanics suggested to take part in fueling NAFLD high prevalence [22] (Figure 4).
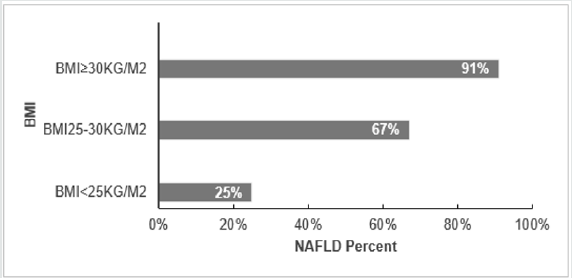
BMI correlates with NAFLD, and it has been revealed as a factor that elevates the probability of emerging NAFLD. In years, 1980-2008, the BMI-mean augmented worldwide by 0.4 kg/ m2 for males and by 0.5 kg/ m2 for females per decade. This coincides with obesity prevalence that increased simultaneously from 4.8 to 9.8% for men and from 7.9 to 13.8% for Women [23]. This factor was investigated in the region of European Union and it’s clear that similarly to other regions, in Europe the higher risk of acquiring NAFLD is elevated coincidently with the increases of BMI, 25% (BMI<25kg/ m2), 67% (BMI 25-30 kg/ m2) and 91% (BMI ≥30 kg/ m2) [24]. Together with other risk factors (obesity or T2DM) which more likely associate to sedentary life, are increasing in Europe fueling the NALFD prevalence and further the impairments such as cirrhosis and HCC [25].
The NAFLD prevalence among adults of sub- region Shanghai was considered to show high growing trend of NAFLD with time, it’s obvious that NAFLD prevalence is elevating with time [26]. The research showed diversity of NAFLD prevalence in different subregions of Asian countries, in Taiwan (15%-27%), Korea (24%- 40%), and Japan (9%-18%) [27]. Additionally, prevalence in India has increased from 28% in 2015 to 31% in 2016 in the rural region of Haryana [28]. Timely elevation of Fatty Liver was also revealed in different sub-regions of China [29]. Shanghai with high rate in development that goes together with life behavioral changing; the incremental in NAFLD is seen to increase sharply in adults in recent years. Findings supported by [30-33], shown in Figure 5.
NAFLD Pathogenesis
Numerous researches have reported that metabolic
impairment or disturbance is the basic abnormality in NAFLD [34].
At the center of this discovered metabolic abnormality there is
insulin resistance, that also known among the initiators of NAFLD.
Thereafter, by provoking oxidative stress, fatty liver may evoke
inflammation and hepatocyte injury which may cause the disease
to tend to NASH and cirrhosis. The overload of triglycerides (TG)
as fat droplets in hepatocytes cytoplasm was reported as the main
event of NAFLD, which is a precondition for the succeeding NASH,
as it was shown by liver biopsy that 5%-10% of hepatocytes have
fat droplets [35]. Increased moving of both TG and free fatty acids
(FFA) to the liver, reduced hepatic using of FFA, decrease in export
of TG from liver, disturbed beta-oxidation of FFA in hepatocytes
result in stockpiling of TG within hepatocytes cytoplasm [34,36,37].
Another main stimulus for liver de novo fatty acid synthesis, is the
surplus carbohydrate from either dietary origins or hepatic de novo
gluconeogenesis [36,37].
Obesity can be defined as a chronic low-grade inflammatory
condition. There are cytokines related to obesity, such as
interleukin-6 (IL-6), leptin, adiponectin, and tumor necrosis factoralpha
(TNF-α) that play a remarkable role in NAFLD evolution.
Research findings reported that adipose tissue may be the inducer of
inflammatory mediators and adipokines, such as pro-inflammatory
ones (IL-6, TNF-α, and leptin) and anti-inflammatory (adiponectin)
effects [38]. Even though, these hormones and cytokines may
ordinally work in balance, the homeostasis is disturbed in NASH
that result in increased TNF-α and reduced adiponectin levels.
Several mechanisms that likely involve in hepatocellular injury
in setting of NAFLD, many of them produce the ROS. A liver with
surplus fat can be more prone to stressors, such as adipokines,
reactive ROS, and cytokines than normal liver [39,40].
In the study done by Yang and colleagues, obese mice with fatty
liver cleared endotoxins less than nonobese controls [38]. Factors
that perform key functions in the evolution of NASH from simple
steatosis remain unclear [41-43]. Some known possible ways are
oxidative stress through increasing ROS and reduced antioxidants,
lipid peroxidation, reactive metabolites, 4-hydroxynonenal and
malondialdehyde, adipose tissue products. Other discovered ways
are Fas-ligand, transforming growth factors-β1, respiratory chain
deficiency along with mitochondrial dysfunction, and intestinal
microbiota by augmented intestinal permeability, elevated energy
gaining from diet, intestinal leak, bacterial lipopolysaccharides
(LPS), TNF-α, and endotoxins [44].
Hallmarks in NAFLD
NAFLD and oxidative stress
Oxidative stress increases the loss of structure and function of healthy cells, DNA and also damage of important macromolecules. These conditions are the main roots for chronic diseases such as cancer, stroke, cardiovascular impairment including diabetes [45]. Oxidative stress has been found to disrupt insulin signaling process, which result in insulin resistance in cell [46]. Yet, impaired insulin signaling mechanism remains unclear [47] (Rains & Jain, 2011). Besides, Elevation of oxidative stress is generally a considerable factor in degenerative diseases, including chronic fatigue neurodegenerative diseases. Moreover, oxidative stress can be suggested to mediates the transition from simple steatosis to steatohepatitis and, disruption of metabolic balance. Furthest, the above cited impairments are the crucial features that lead to steatohepatitis in lipid-laden hepatocytes [48]. Oxidative stress arises due to the lower antioxidant capacity and/or overproduction of the ROS [47]. Various mechanisms by which these two scenarios initiate oxidative stress in organism are described in the following paragraphs.
Several factors initiate oxidative stress in diabetes. The main
origin of oxidative stress is mitochondria. Mitochondria use around
98% inhaled oxygen, from which 0.2-2% produce ROS [47,49]. A
part of the used oxygen is reduced to water, and the left oxygen
converted to oxygen free radicals through oxidative metabolism of
mitochondria [50]. Oxygen is required for human life, even though,
it may damage and kill the cells when it produces ROS [51]. The
ROS are engendered by the reduction of molecular oxygen or from
oxidation of water to give products such as superoxide anion,
hydroxyl radical, hydrogen peroxide [47]. The hyperglycemia
promotes low density lipoprotein (LDL) peroxidation and followed
by the production of free radicals [52,53]. The other significant
factor in the production of free radicals is glucose oxidation. In
its enadiol form, glucose become oxidized in a transition-metal
dependent reaction to give enadiol radical anion transformed
into reactive radicals [54]. Other mechanism of oxidative stress
in diabetes is the engendering of advanced glycated end products
(AGEs) [55]. AGEs resulted from the covalent binding of the ketone
or aldehyde groups of reducing sugars, in their way to free the
amino groups of proteins [47].
Alternatively, Antioxidant may be defined as a substance that
delay or inhibit the oxidation of substrate, this scenario comprises
many mechanisms pointed out in oxidative stress production
pathways. There exist various exogenous and endogenous
components which can perform a considerable function in
antioxidant defense and help in prevention of oxidative stress in
organism including Catalase (CAT), glutathione peroxidase (GPx),
superoxide dismutase (SOD) [56]. The alteration of endogenous
antioxidant defense comes in case of hyperglycemia. In diabetes,
both decreases and increases in the activities of major antioxidant
enzymes like CAT, SOD, Glutathione reductase (GR), GPx have
been noticed [57]. Studies have shown that in pancreatic islets
8-hydroxy-2-deoxyguanosine (8-OH-dG) and 4-hydroxynonenal (4-
HNE) levels augmented which support that hyperglycemia might
be a key inducer of oxidative stress in the β-cell and oxidative
stress induced by glucose [58]. The β-cells of pancreatic islets are
vulnerable to the genesis of ROS and the reduction of antioxidant
enzymes activities [59]. In Goto-Kakizaki (GK)-rats, the levels of
8-OH-dG and 4-HNE were high in β-cells of pancreatic islets [60].
Many reports have elucidated that the increasing of peroxide
content in tissues, plasma, and red blood cells of animals that have
chemically induced diabetes [61,62].
Read More about Lupine Publishers Journal of Gastroenterology and Hepatology Please Click on below Link: https://currenttrendsingastroenterology.blogspot.com/

No comments:
Post a Comment
Note: only a member of this blog may post a comment.