Lupine Publishers| Drug Designing & Intellectual Properties International Journal (DDIPIJ)
Abstract
Arylated 5-hydroxy-pyrrol-2-ones were prepared in 2 synthetic steps from muco-chloric acid and were optimised as CCK / CCK2 selective ligands using radio labelled binding assays. A potent CCK selective ligand was identified (PNB-081: CCK1=20nM) as well as a potent CCK2 ligand (PNB-001, IC50= 22nM) during a systematic SAR optimisation. The antagonism was confirmed for both ligands by using isolated tissue preparations with CCK5 and CCK8S. Subsequent in vivo evaluation revealed analgesic activity for the gastrin CCK2 antagonist PNB-001, in the hotplate and tail immersion test at 0.5mg /kg by IP administration in mice. The cholecystokinin antagonist PNB-081 potentiated the analgesic effect of morphine and reversed opiate tolerance in mice from doses >1 mg/kg by oral administration.
Keywords: Phenyl-Pyrolone; CCK Antagonist; Cholecystokinin; Analgesic; Opiate Adjunct
Abbrevations: GIT: Gastrointestinal Tract; TT: Test Thresholds; BT: Base Line Withdrawal Thresholds; ANOVA: Analysis Of Variance
Introduction
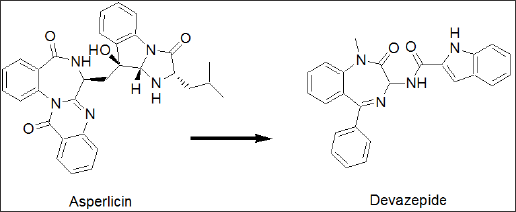
In terms of cholecystokinin-physiology [1], CCK8is the most common peptide hormone, which is extensively found throughout the gastrointestinal tract (GIT) and is also widely distributed through the nervous system [2]. Originally, cholecystokinin was discovered to cause contractions of the gallbladder [3]. It was then rediscovered as pancreozymin, triggering the release of pancreatic enzymes. Finally, it was confirmed that both peptides are identical [4]. Cholecystokinin acts as a neuro modulator as well as gut hormone. CCK-ligands, agonists and antagonists have been extensively investigated as potential drug molecules [5]. Cholecystokinin antagonists have been extensively investigated as potential drug targets [6]. They were studied as growth inhibitors in certain forms of cancer [7], as anxiolytics [8], in the treatment of schizophrenia [9] and satiety [10]. An agonist, the shortened CCK4 was found to induce panic in patients [11] and the CCK2 receptor is known to mediate anxiety [12] and panic attacks [13]. Cholecystokinin does cause proliferation in colon- and pancreatic cancer cell lines and therefore, CCK-antagonists were studied as growth factor inhibitors in certain forms of cancer. Asperlicin was the first non-peptidal lead structure from nature [14] and analogues thereof were studied as CCK ligands [15]. Simplification of this lead structure by Merck led to Devazepide [16], a potent CCK1 selective cholecystokinin antagonist (Figure 1a), containing a 1,4- benzodiazepine template and an indole moiety.
Figure 1a: Discovery; Merck's CCK patents. From the natural product asperlicin to a fully synthetic 1,4-benzodiazepine lead structure.
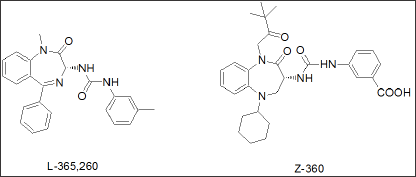
Proglumide [17] was the first glutamic acid based agent, marketed as Milid for the treatment of ulcer. Lorglumide, a derivative of proglumide [18], (Figure 1b) is one of several CCK receptor antagonists [19] and served as experimental standard. The indolyl amide of devazepide was replaced by a urea linkage and Merck's L-365,260 resulted in a CCK2 selective antagonist [20]. Further subsequent SAR optimization led to Zeria's improved Z-360 [21], in which the N-alkyl side chain, the 5- position (cyclohexyl) was optimized for potency and a meta-carboxylic acid on the aryl urea linkage was introduced to enhance water solubility (Figure 1c). Z-360 is a CCK2-gastrin receptor antagonist and progressed into phase 2 trial with pancreatic cancer [22]. Z-360 is the most recent derivative derived from this original lead structure, with improved selectivity and bioavailability.
Figure 1b Cholecystokinin standard-Lorglumide. Rotta Research Labs. Patents based on glutamic acid, agents with little membrane penetration.
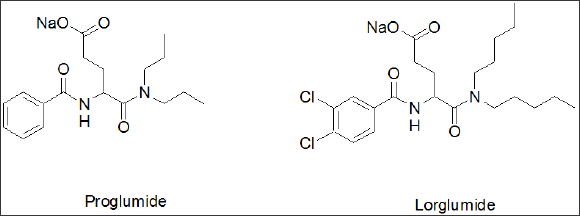
Figure 1c: Merck's SAR optimisation from Devazepide towards a gastrin antagonist L-365,260 with final optimisation by Zeria Ltd and selection patent on Z-360.
All structural optimisations did only partly address the main underlying problem with respect to poor pharmacokinetic properties, such as a low water solubility and very low membrane penetration, as a result of a large polar surface area of the molecules and a relatively high molecular weight. In our early search for new CCK ligands, in which the 1, 4-benzodiazepine structure [23] was replaced by an achiral diphenyl pyrazolone template, novel CCK antagonists with an indole carboxylic acid [24] and a phenyl urea moiety [25] were found and optimized with excellent animal data on anxiety and depression [26]. The patent was discontinued as structure was part of a Chinese molecular modelling patent application (Figure 1d). The 1,4-benzodiazepine template was varied by a combinatorial solid phase synthesis [27] and it was SAR optimised in terms of CCK binding affinity to a benzodiazepine with a simple propyl group [28]. Again, having realized the poor pharmacokinetic properties these agents, a search for a completely novel, smaller template with a molecular weight <350, a log p about 3 and a polar surface area for membrane penetration of less that 100A, with no urea linkage was initiated. Aim of the drug discovery programme, initiated by PNB Vesper Life Sciences, was to systematically investigate and design the 2(5H)-furanone scaffold [29] in to a hydroxy-pyrolone scaffold with ligands for both CCK pathways (Figure 1e). Molecular pain targets have been reviewed recently [30] and the results are quite disappointing in terms of efficacy and FDA approval rate. Even, this review missed out on CCK antagonists [31] and most importantly on a very positive report, publicised only in form of an abstract [32]. In summary in this study, devazepide at 5 mg was found very efficient in pain management as adjunct to strong opiates in a phase 2 trial carried out at leading UK pain research centres. Initial results for CCK antagonists of the pyrolone scaffold were communicated in the area of cancer therapeutics [33] and GI inflammation [34]. Here, a full biological evaluation of PNB Vesper Life Science's pain moleculesPNB-001& PNB-081, which are covered by an original patent, are reported in detail here with respect to pain management [35].
Figure 1d: Urea / Amide based CCK antagonists covered by Aston University patent replacing the 1,4-benzodiazepine scaffold with a pyrazole.
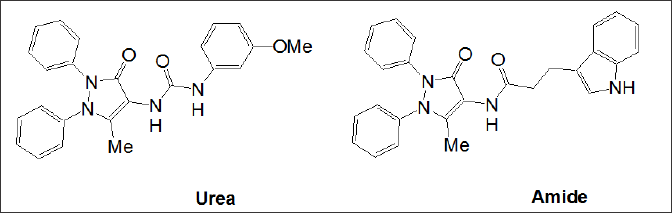
Figure 1e: From lactones to lactams. Discovery of pyrolones as a novel CCK-template. From an Aston University user patent on 4-amino-2(5H)-furanonesto a novel original template.
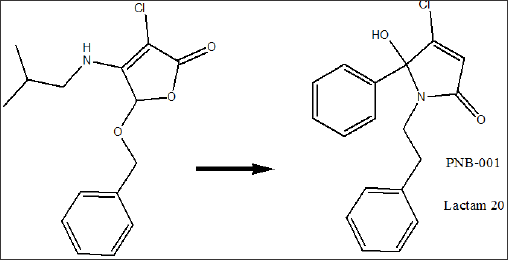
Results and Discussions
Chemistry
5-arylated dichloro-2(5H)-furanones A and B were synthesised from muco-chloric acid (Scheme 1), which is commercially available from furfural under oxidising conditions with hydrochloric acid. Theses intermediates were evaluated previously as anticancer agents [36]. Muco-chloric acid was reacted with benzene as reagent and solvent at RT under the development of hydrogen chloride gas. Depending on the scale of the reaction cooling with ice was required. For chloro benzene / benzene the powdered or most preferred granulated aluminium chloride served as the best catalyst and during work up with hydrochloric acid on ice the inorganic salts were easily removed from the organic phase. In terms of scope of the reaction arylated 2(5H)-furanones, containing nitro- groups or trifluoro-methyl groups could not be prepared. For the small scale synthesis aluminium chloride worked well as Lewis acid. However, during scale up aluminium chloride was replaced by trifluoroborane in THF as the exothermic reaction become problematic on a kg scale.
Scheme 1: Synthesis and chemical mechanism for the preparation of lactams1-21 from muco-chloric acid.
a) Arene, RT, 10 h, workup: hydrochloric acid; b) amine , excess, ether, RT, 30 min
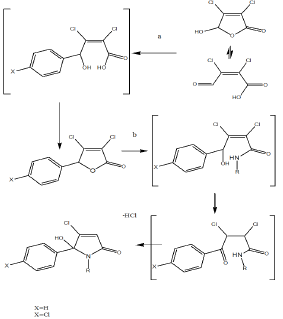
Subsequent reaction of the 5-arylated 3,4-dichloro-2(5H)- furanones A and B (Stage 1 intermediate) in diethyl ether with alkyl- and aryl alkyl amines furnished N-alkylated hydroxyl-pyrrolones1- 21(Stage 2 products) in high yields under mild conditions. The general synthetic sequence is outlined in Scheme 1.
Overall, the desired N-alkylated unsubstituted 5-phenyl pyrrolones1-21 was obtained in only a 2 stage process as white crystalline material. The molecule is not present in the ring opened keto form and fully occurred in the 5-membered ring form, as a hydroxy-pyrolone. The 5-arylated 2(5H)-furanones reacted selectively in the ester position and no reaction in the 4-position was observed here. Previously the IPSO substitution (4-position) was described for pseudo- esters [37], and here in Scheme 1, a ring- opening ring-closure mechanism is proposed for the formation of hydroxy-pyrolone. Thus, the first step in the reaction sequence of the dichlorinated 2(5H)-furanone is the ring opening and amide formation from the corresponding lactone. Subsequently, the keto form of the acyclic amide was in situ converted into a lactam under the elimination of hydrogen chloride (middle, scheme 1). The analysis of lactam7 and 20by chiral HPLC showed a 50:50 racemic mixture of both enantiomers in solution in methanol.
Read More About Lupine Publishers Drug Designing & Intellectual Properties International Journal (DDIPIJ) Please Click on Below Link: https://lupine-publishers-drug-designing.blogspot.com/

No comments:
Post a Comment
Note: only a member of this blog may post a comment.