Lupine Publishers| Journal of Drug Designing & Intellectual Properties
Abstract
Early anti-cancer research was dominated by the development of alkylating agents, followed by the discovery of a variety of anti-metabolites, which were useful anti-viral agents at the same time. Current anti-cancer drugs are designed towards molecular targets in order to reduce their toxicity and to enhance the selectivity on the cancer cells. Within an increasingly growing number of molecular targets, the cholecystokinin, as a neuro modulator, became an important anti-cancer target, especially when it was shown that cholecystokinin regulates the invasiveness of human pancreatic cancer cell lines via the protein kinase C pathway. The low potency and the lack of subtype receptor selectivity of those early non-peptide CCK-antagonists, was improved in the following generations of CCK antagonists. These potent and selective antagonists have shown disappointing results in clinical trials due to a poor bioavailability. Initially cholecystokinin was discussed as growth factor, not only in pancreatic cancer, but also for lung, breast, colon and brain cancer, followed by a detailed discussion of over 20 different chemical classes having been developed to date, mainly for the area of neuroscience. Loxiglumide, CI-988, Devazepide, L-365,260 and YM022 are highlighted including in vivo studies and clinical trials. Moreover, CCK antagonists were found useful in the enhancement of the analgesic effects of morphine and the anti-neo plastic effect of cis-platinium. Clinical trials are ongoing. It is concluded that non peptidal cholecystokinin receptor antagonists are modern, non-toxic anti-cancer agents.
Abbrevations: GI: Gastrointestinal: Bt2cGMP:Dibutyryl Cyclic Guanosine Mono Phosphate; Bt2cGMP:Dibutyryl Cyclic Guanosine Mono Phosphate; CCK: Cholecystokinin
Introduction
Several gastrointestinal (GI) hormones, such as gastrin, cholecystokinin, and bombesin, have been reported to affect the development of pancreatic cancer. The receptors for these hormones are found in normal and neo plastic pancreatic cells. Activation of these receptors enhances pancreatic carcinogenesis and promotes the growth of established pancreatic carcinoma either in vitro or in vivo. Studies have shown that these GI hormones may play an inhibitory role in the development of pancreatic cancer. In recent years, increasing emphasis has been placed on the effects of GI hormones on cancer invasion and metastasis. As the transition from non-invasion to the invasive state is the crucial event in cancer development, further investigation of the way in which GI hormones affect the invasion and metastasis of pancreatic cancer may be important for the development of new therapeutic approaches with eventual clinical utility [1].
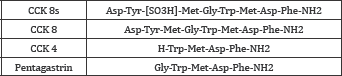
Cholecystokinin (CCK) is produced by I cells of the duodenal and jejunal mucosa and exists most prominently as an eight amino- acid hormone (CCK-8). CCK has been long been recognized as having an effect on the regulation of pancreatic secretion [2] and of gall bladder contraction [3]. Cholecystokinin has also been found in the brain, where it is widely distributed and may therefore have an effect as a neuromodulator or perhaps as a neurotransmitter. CCK is characterized by the a -aminated terminus Trp-Met-Asp- Phe-NH2 aminated sequence. It was initially identified as a 33 amino acid chain [4] and was later synthesized [5]. Subsequent studies have revealed the existence of multiple forms [6,7]. CCK is derived from a primary prepro-CCK polypeptide of 115 residues. After transcription, enzymatic cleavage results in the formation of many different fractions. CCK58, CCK39, CCK33, CCK22, CCK8s (sulphated), CCK8ns (non-sulphated), CCK7, CCK5, CCK4 all of them demonstrate biochemical activity [8]. The predominant circulating form is a sulphated tyrosine residue at position 7. It is important to distinguish between the CCK tetra peptide [9] and octapeptide (Sincalide) [10] as shown in Table 1. Both of them have been extensively studied, particularly in relation to food intake regulation, and have brought a great deal of confusion when it came to anxiety and panic. They have differential affinity for CCK receptors [11,12] different distribution in both the periphery and the brain [13,14] and have various effects on behavior.
Table 1: Amino acid sequence of Cholecystokinin and Penta gastrin fragments.
Cholecystokinin and Cancer
Cholecystokinin (CCK) plays an important role in the invasiveness and the production of matrix metalloproteinase-9 (MMP-9) in human pancreatic cancer cell lines. The pathway of the invasiveness may be associated with MMP-9 of those lines regulated by CCK. Two human pancreatic cancer cell lines were treated with CCK-8 alone, CCK-8 and staurosporine, or CCK-8 and indomethacine. The invasiveness and the production of MMP-9 were decreased with staurosporine but not indomethacine. These results suggest that CCK may regulate the invasiveness and the production of MMP- 9 via protein kinase C in human pancreatic cancer cell lines [15]. Cholecystokinin (CCK) receptors play a role in the development and growth of pancreatic cancers. The expression of mRNA encoding CCK-A and CCK-B receptors in eight human pancreatic tumour cell lines was detected using reverse transcription-polymerase chain reaction (RT-PCR), but not by RNase protection assays. The K-ras gene, which can be activated by G-coupled protein receptors such as CCK receptors, was mutated in codon 12 in five of the cell lines. In addition, Mia PaCa-2 pancreatic cancer cells did not respond to CCK or gastrin in cell proliferation or focal adhesion kinase (FAK) phosphorylation assays. In contrast, mouse NIH3T3 fibroblasts transfected with human CCK-B receptor (NIH3T3CCK-BR) showed increased proliferation and phosphorylation to the peptides [16].
The gut hormone cholecystokinin exerts various actions on the gastrointestinal tract, including the regulation of growth. The hormone has been reported to induce hypertrophy and hyperplasia of the pancreas and to enhance chemically-induced pancreatic carcinogenesis in animals. Stimulation of endogenous cholecystokinin secretion through the induction of deficiency of intra intestinal proteases and bile salts by trypsin-inhibiting nutrients, bile salt-binding drugs or surgical intervention is also capable of stimulating growth and tumor development in the rat. In man, factors suggested to increase the risk of pancreatic cancer, such as a high-fat and high-protein diet or gastrectomy, are known to stimulate plasma cholecystokinin secretion. Receptors for cholecystokinin have been demonstrated on human pancreatic adenocarcinomas, and cholecystokinin has been demonstrated to enhance the growth of xenografted pancreatic cancer and to inhibit growth of gastric and bile duct cancer [17].
From CNS Drugs to New Anticancer Agents
Gastrointestinal polypeptide hormones regulate growth of various normal gastrointestinal tissues as well as certain visceral cancers [18]. If Cholecystokinin promotes cell growth, CCK antagonists are ideal chemical anti-cancer targets and many scientists have discovered specific peptide and non-peptide antagonists of CCKB/gastrin receptors up to date, mainly for the area of neuroscience. As a result of extensive research, a number of new chemical classes have been developed with a high potency and selectivity towards the cholecystokinin receptor subtypes.
Amino Acid Derivatives
Figure 1: Structures of early amino acid derivatives as CCK antagonist.
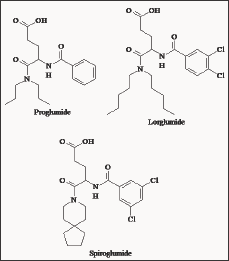
During the 1970's amino acid derivatives (Figure 1) were found to contain anti gastrin activity [19,20]. The chemical similarities of gastrin and CCK made it possible for such derivatives to demonstrate CCK antagonist activity. Proglumide, the first putative gastrin antagonist clinically available, has long been used in the treatment of peptic ulcers, because of its anti secretory and gastro protective activities. Several studies have subsequently demonstrated that proglumide is also a weak CCKA receptor antagonist [21] and despite its low potency, it has been the reference CCK and gastrin antagonist for several years.
Rotta research group produced analogues of proglumide, which showed varying degrees of selectivity for CCKA receptors and even suggested possible sub-types of the peripheral receptors. Some derivatives had a higher affinity for pancreatic CCK receptors mediating gallbladder contraction. Lorglumide showed up to a 26-fold increase in potency for blocking CCK-stimulated gallbladder contraction but only a two-fold increase for blocking CCK-stimulated pancreatic amylase secretion [22]. Intravenous administration of Lorglumide [23] antagonized the CCK-induced reduction of gastric emptying in rats, acceleration of intestinal transport in mice, increase in ileal motility in rabbits, gallbladder contraction in guinea pigs and acceleration of gallbladder emptying in mice but showed reduced activity when orally administered. Further structural modifications to Lorglumide resulted in CR2194 (spiroglumide). Spiroglumide exhibited CCKB/gastrin antagonist in the micro molar range, with excellent oral bioavailability. However, it has poor selectivity for CCKB/gastrin receptor, which raises doubts of its potential therapeutic usefulness. The effect of loxiglumide (LXG) was studied on the invasiveness of two human pancreatic cancer cell lines. Cells were treated with LXG for 24 h, and examined in the invasion assay. Interestingly, the invasiveness of cancer cells and expression of MMP-9 were decreased by LXG in a dose-dependent manner [24].
Read More About Journal of Drug Designing & Intellectual Properties Please Click on Below Link:
https://lupine-publishers-drug-designing.blogspot.com

No comments:
Post a Comment
Note: only a member of this blog may post a comment.