Lupine Publishers | Journal of Organic and Inorganic Chemical Sciences
Abstract
In this work, a series of active Au/NiOx catalysts were successful to prepare by tracing the concentrations of chloride in the re-dispersed aqueous solutions. By characterizations, we found that the appropriate amount of residual chloride in Au catalyst would induce Au nanoparticles (Au NPs) to locate on the edges of NiOx particles, which resulted in the active Au/NiOx-9 sample. Fine control of chloride in the aqueous solution provides a new perspective to push for addressing the controllable preparation of active heterogeneous catalysts.
Keywords: Au catalyst, Preparation, Chloride, Esterification
Introduction
In recent decades, Au catalysts have received growing attentions and been widely applied in many important research fields [1], since good performance of Au catalysts was discovered [2]. However, the controllable preparation of highly active heterogeneous catalysts is still a longstanding challenge till now, especially Au catalysts. Many efforts have been devoted to this problem. The active site, structure and the quantum size effect of Au catalyst [3], active oxygen species of the support [4], suitable reducible oxide supports [5],and so on, have been extensively studied. Additionally, catalyst precursors, bases, pH value, aging time, and calcinations temperature are also crucial conditions [2,6]. Nevertheless, the controllable preparation of highly active Au catalyst is still difficult to realize even strictly following all above conditions. Chloride (usually as Cl-) is generally regarded as a poison for Au catalyst, Because of strong interaction of chloride and Au. We realized the reproducible preparation of Au/Fe2O3 catalyst for CO oxidation [7]. It is meaningful to explore whether this method can be applied to other catalysts and reactions or not. In this work, Methyl esterification of alcohols was chosen as model reaction. The controllable preparation of highly active Au/ NiOx catalyst was realized by tracing the concentrations of chloride in the re-dispersed aqueous solutions.
Experimental Details
Au/NiOx catalyst preparation
20ml Ni(NO3)36H2O (0.011 M) and 1.05 ml HAuCl4 (0.24M) were mixed together and were drop wise added into 60 ml Na2CO3 solution (0.31M) under vigorous stirring in 3h. The turbid liquid was divided into four sections and separation by centrifugation. Each section of the recovered precipitate was re-dispersed in different amount of deionised water and ultrasonically washed for 1h. The chloride concentration in the re-dispersed aqueous solution of each section was determined by CHI660D electrochemical workstation. Then, the solid was separated by centrifugation, dried at 80o C for 3h and calcined at 350 oC for 0.5 h to produce the catalyst sample, which was denoted as Au/NiOx-X, in which X suggested the chloride concentration in ppm.
Catalyst activity test
1mmol benzyl alcohol, 30 mg catalyst and 2 ml methanol were added into a glass tube. And then it was exchanged with oxygen and reacted at 60o C (1 atom, O2 balloon). After reaction, it was cooled to room temperature. Biphenyl was used as internal standard and a certain amount of ethanol were added into the reaction mixture up to 10mL for quantitative analysis by GC-FID (Agilent 7890A).
Results and Discussion
The catalytic activities of 15 Au/NiOx samples, which were prepared from the re-dispersed aqueous solutions with chloride concentrations in the range of 2 to 108 ppm, for esterification of benzyl alcohol were studied. According to the results shown in Figure 1, catalytic activity of Au/NiOx varied with the changing of chloride concentration. The yields of methyl benzoate were lower than 21% if the catalysts were prepared from aqueous solutions containing >22ppm chloride. More active catalysts were produced when the chloride concentrations were going down. The Au/ NiOx catalysts with the highest catalytic activity were prepared from aqueous solutions containing 8-13ppm chloride, the yield of methyl benzoate of catalyst Au/NiOx-9 was >99%. Surprisingly, the catalysts turned less active again when the chloride concentrations were < 8ppm. Typically, the yield of methyl benzoate was 20% with catalyst Au/NiOx-3.
Figure 1: The yield of methyl benzoatevs the chlorine concentration of the aqueous solution from which the catalyst samples were prepared.
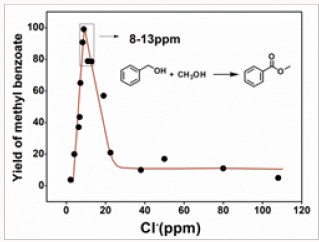
Figure 2: HR-TEM (left) images and size distributions (right) of Au/NiOx-22 (a), Au/NiOx-9 (c), and Au/NiOx- 3(e).
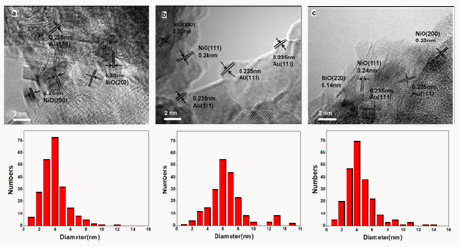
TEM measurement results of Au/NiOx are shown in Figure 2. Their TEM images were similar and seemed amorphous. For the sample of Au/NiOx-22, the lattice of gold could be observed and wrapped in NiOx particle. For active Au/NiOx-9, the most of Au NPs connected with the edges of NiOx particles or the junctions of several NiOx particles [8]. In consideration of the best catalytic performance of this sample, this observation strongly supported the former results about active site in Au catalyst, i.e. the interface between Au and iron oxide [3]. It suggested that the appropriate amount of chloride might act as the linkage between Au NPs and the edges of NiOx particles to gain the active Au catalyst, For Au/ NiOx-22 and Au/NiOx-3, too much or less chloride was presented, the interaction of Au NPs and NiOx like Au/NiOx-9 decreased significantly. Accordingly, the catalytic activity lost sharply. By metering more than 150Au NPs, the mean diameters of Au NPs in samples Au/NiOx-3, Au/NiOx-9 and Au/NiOx-22were 4.1, 3.8 and 6.6 nm with 1.91, 1.84 and 3.06 standard deviations. The size distributions of Au NPs in Au/NiOx-3 and Au/NiOx-9 samples were extremely similar. The marked difference of catalytic activities of these two catalysts did not come from the size effect of Au particles, but the contact way of Au NPs and NiOx supports.
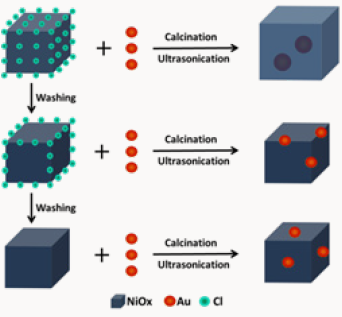
At present, there is still not sufficient evidence to explain the real role of chloride in the formation of Au catalysts. However, according to the known evidence, we can make some reasonable conjectures. Firstly, as pH value of the mother aqueous solution rises, chlorine in chloroauric acid is substituted by the hydroxyl. Au-Cl bond breaks and then small Au NPs form. Finally, chloride is adsorbed on the support NiOx as well as Au NPs. Due to the stronger interaction of chlorideon the edges than on planes of NiOx crystallites, after the ultrasonication and washing operations, chloride located on the edges of NiOx crystallites remains. As shown in Figure 3, it is this kind of residual chloride that induces Au NPs to anchor on the edges of NiOx crystallites.
Conclusion
In summary, by tracing the chloride concentrations in the re-dispersed aqueous solution, we successfully prepared active Au/NiOx catalyst for catalytic methyl esterification of alcohols. If the chloride concentration was not in the range of 8-13ppm, the catalytic activity dropped dramatically. These results indicated that the presence of appropriate amounts of residual chloride was beneficial to obtain highly active heterogeneous catalysts. This work can offer a new perspective to realize the controllable preparation of active heterogeneous catalysts.
Read More About Lupine Publishers Journal of Chemistry Please Click on Below Link:
https://lupinepublishers-chemicalsciences.blogspot.com/

No comments:
Post a Comment
Note: only a member of this blog may post a comment.