Lupine Publishers| Journal of Material Science
Abstract
The present investigation deals with the determination of the physio-chemical properties of two commercial grade samples of local gums (Gum Arabic and Natural Rubber Latex (NRL)). The results revealed that the gum samples have high melting point that indicate thermal stability at room and moderate temperatures. The gum samples have about 95 % carbohydrate content and a corresponding high internal energy and can serve as a source of energy. The rheology of the samples revealed shear-thickening characteristics with gum Arabic being thixotropic and pseudo-plastic in nature while NRL was observed to be anti-thixotropic and rheopectic. Further results from the moisture absorption, contact angle and Fourier Transform Infrared Radioscopy (FTIR) analyses gave better insight into their hydroscopic behaviours. Gum Arabic has excellent water absorption capacity with less wettability as it consists mainly of more water-soluble compounds in comparison to Natural Rubber Latex. These insights from the study will enhance wider application of the gums with increased value-addition to the gums and the communities where they (can) thrive.
Keywords: Gums; Density; Wettability; Latex; Gum Arabic; Rheology
Introduction
Rubber latex and gum Arabic are gums which are water-soluble
polysaccharides that are extractable land plants. They also contain
some protein materials and are important agro-forestry resources
in Nigeria [1]. They can enhance the viscosity and gelling ability
with their dispersions. Hence, the key qualities of the gums are their
water solubility and high viscosity in aqueous dispersions. Guar
gums are harvested from the stems and branches of the resource
gum trees as dry exudates. Hydrophobic affinity chromatography
showed that gum Arabic is made up of three major constituents
namely, Arabinogalactan (AG), Arabinogalactan protein (AGP),
and Glycoprotein (GP). The highly branched polysaccharide
part of the gum represents about 90% of the total gum [2]. The
complex nature of plant gums is since each gum sample possess
a special combination of sugar (monosaccharide unit) to form the
polysaccharide. The most widely distributed sugar in plant gums
are mannose, galactose, ramose, fructose, xylose, and the sugar
acid. They contain residual amount of fats, protein, metabolites,
metal ions, crude fibres etc. Raw materials of plant origin
have proven to be relatively non-toxic, bio-compatible, readily
accessible, economical, and cost effective. In this regard plant gums
are used for wide industrial applications such as in the cosmetic,
pharmaceuticals and in food industries [3].
The prediction of the properties of these gums is a challenge
because of their heterogeneity in addition to their complex nature.
Hence, their industrial applications require reliable information
that can only be obtained from proper characterization of
samples. The physicochemical properties of a compound are the
measurable physical and chemical characteristics by which the
compound may interact with other systems. This characteristic
collectively determines the quality, applicability or the end-use
of the compound. In plant gums, these properties are directly
influenced by the botanical type, age, location, nature of the
growing soil and the climatic condition around the resource gum
tree [1]. Physicochemical characterization of gums therefore is
an essential step towards establishing suitability for industrial
application. This focus of this study is to determine the physio-chemical properties and characterization of natural lubber
latex and guar gum as found in Nigeria. This is to enable us gain
insight into their quality, applicability, and end use in industries.
Information about these properties are scanty in open literature.
Such physio-chemical properties include the ash content, moisture
content, moisture absorption, pH, percentage lipid content, crude
protein, carbohydrate content, and functional groups, among
others. Just as other natural resources have been studied (Abdallah,
Edomwonyi-Otu, Yusuf, & Baba, 2019; Edomwonyi-Otu & Aderemi,
2010; Edomwonyi-Otu, Aderemi, Ahmed, Coville, & Maaza, 2013;
Gimba & Edomwonyi-Otu, 2020) The knowledge of their properties
and applications for value addition and enhance the economic
wellbeing of the communities where they thrive.
Experimental
Preparation of Samples
Gum Arabic: The crude sample consisted of mixture of large and small nodules mixed with the bark and other organic debris obtained from the bark of wounded Acacia Senegal plant and Frankincense plant. Hand picking method was used to separate the neat gum lumps from debris and other constituents. The lumps were then spread out under room temperature to dry. The dried sample was then ground into powder and 150 g of the sample weighed inside an empty dried weighed bucket by using the weighing balance (CWS Series with accuracy of ±0.05% FS). The sample was added to a 200 ml hot boiled water to dissolve the sample. Proper protective equipment was worn for safety purposes. The gumwater system was properly stirred to ensure complete dissolution of the gum in water. It was thereafter stored in a tight container at room temperature for 48 hours and stirred at 6 hours intervals to ensure a perfect dissolution, proper hydration and adsorption (Edomwonyi-Otu, Chinaud, & Angeli, 2015). The dissolved solution was centrifuged (strained) to remove air bubbles and any insoluble residual lumps through a muslin cloth (mesh) in a jug and sieved into another container. The undissolved lumps of the sample were transferred into a crucible and placed in the oven (Gallenkamp TM OV-420) at a temperature of 90 ̊C to dissolve further by gentle heating. The dissolved solution was then mixed, weighed and filtered using a mesh to separate the clear gum solution from dirt. The weight of the clear solution after filtration was measured. Formalin was added to the gum Arabic clear solution to prevent deterioration, and then stored[1].
Natural Rubber Latex: 300 ml of the crude rubber latex gum was tapped from the stem of rubber latex plant (hevea brasiliensis) using a v-shaped knife. The raw latex was then centrifuged to remove water molecules. Ammonia solution was then added to the solution to prevent deterioration [4].
Analysing the Physio-Chemical Properties of The Gum Samples
Density and Specific Gravity Measurement
Density and specific gravity analysis of 1% w/v of the test samples was carried out using a density bottle and following methods described elsewhere [5]. The weight of the empty bottle was measured (W) using CWS Series weighing balance (accuracy of ±0.05% FS) and then weight of the bottle with 70ml of the test samples of gum acacia solution and rubber latex gum respectively as (W1). The difference was taken to obtain the actual weight of the liquid solution; the weight was divided by the volume to obtain the density as shown in equation (1)
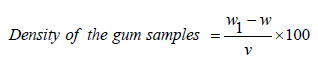 (1)
(1)
Where, W1 is the weight of bottle and the test sample of the gum solution, W is the weight of the empty bottle and V is the volume of the gum samples.
Ash Content and Moisture Content
The analysis of moisture and ash content was determined using 2 clean crucibles of known weight dried in an oven at 102 ̊C for 30 minutes. 10g of both samples were placed in the crucibles and the put into an oven (Gallenkamp TM OV-420) at a regulated temperature of 125 ̊C for 6 hours. The Moisture content was taken as percentage ratio of the change in the weight to the original sample weight. The dry weight of the ash was taken, and the ash was ignited at 550 ̊C in a muffle furnace for 1 hour, content was cooled in a desiccator for 30 minutes and weighed. The ash content was taken as the percentage loss in weight after ignition to that of the original sample. Equation (2) and (3) gives the temperature for calculating % moisture and % ash content respectively.
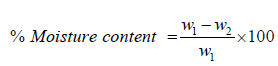 (2)
(2)
Where; W1 is the original weight of the gum samples, W2 is weight of the gum samples after drying
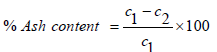 (3)
(3)
Where; C1 is the dry weight of ash, C2 is the weight of ash after ignition
Read More About Lupine Publishers Journal of Material Science Please Click on Below Link:

No comments:
Post a Comment
Note: only a member of this blog may post a comment.