Lupine Publishers | Journal of Food and Nutrition
Abstract
This study evaluated the quality of honey imported from eighteen different countries into Saudi Arabia. Twenty pesticides, 5-hydroxymethlfurfural and the antibiotic chloramphenicol were analysed. Approximately 20% of honey was rejected out of 712 consignments. Ten countries breached the regulations for one or more of the following: exceeding the MRLs, using banned pesticides or presence of chloramphenicol. Three neonicotinoids; acetamiprid, imidacloprid and thiamethoxam was found in combination with other pesticides. The HMF content of honey from eight exporting countries exceeded 80mg/kg. Despite the years of monitoring for pesticides, breaches of MRLs continue to be reported. Recommendation for more stringent approaches to the management of pesticide along the supply chain are suggested as the implications to bee pollinators, environment and human life are wide, varied and unsafe.
Keywords: Pesticides; Monitoring; Honey; Saudi Arabia
Introduction
Honey is defined by Codex Alimentarius [1] as the natural sweet substance produced by honey bees from the nectar of plants or from secretions of living parts of plants or excretions of plant sucking insects on the living parts of plants, which the bees collect, transform by combining with specific substances of their own, deposit, dehydrate, store and leave in the honey comb to ripen and mature. The demand for natural sweeteners is on the increase globally [2-3] and many consumers prefer honey since it has a multitude of uses and benefits [4-5]. In Saudi Arabia, and in many other countries, the major motivators for consuming honey include health and wellbeing, medicinal and nutritional value [6]. However, bee products can also be a source of toxic substances [7-8]-antibiotics (such as chloramphenicol) [9], pesticides (neonicotinoids) [10] and heavy metals (e.g lead, cadmium and arsenic) [7] due to environmental pollution and misuse of beekeeping practices. Pesticide residues have been implicated in genetic mutations and cellular degradation while the presence of antibiotics may increase resistance in human or animal pathogens. In addition, Abeshu and Gelata [6] reported there have been cases of infant botulisms that have been attributed to contaminated honey. Honey that has not been analysed and sterilized should not be used in infants and should not be applied to wounds or used for medicinal purposes. The Maximum Residue Limit (MRL) set for neonicotinoids by the European Union Commission are 50ng/g for acetamiprid, imidacloprid and thiacloprid and 10ng/g for clothianidin and thiamethoxam [11]. Due to their high acute toxicity and concern, the European Food Safety Authority re-assessed the risks and placed a moratorium in 2013 on three [12-14] of the most harmful neonicotinoids (imidacloprid, clothianidin and thiamethoxam). The poisoning of bee pollinators is a result of serious adverse effect of insecticide use, which leads to a drastic decrease in the insect numbers, reduction of honey yields, destruction of plant life, presence of insecticide residues in food, and ultimately, to significant losses in the income of beekeepers. Thus, the main purposes for monitoring bee products are to assist in public health protection, global commercial competition and to realise better quality products. In addition, this provides a greater understanding of some of the issues in the supply chain with regard to pesticide loads as bee pollinators have been recognised as bioindicators of environmental pollution [15].
Saudi Arabia imported US$73 million worth of honey and the worldwide importation of honey totalled US$2.01 billion in 2019 [16]. In international trade, the quality of honey will vary depending on a number of factors. These may include the authenticity (nature, organic, region etc) type of honey (blossom honey, honeydew honey, comb honey, filtered honey, bakers honey etc), moisture content, electrical conductivity, diastase activity, 5-hydroxymethylfurfural (HMF) content, antibiotics, colour and sugar content (glucose and fructose together and sucrose). According to Codex Alimentarius Standard [17] these quality standards are not compulsory for governments and can be voluntarily agreed upon, while according to the EU draft they have to be fulfilled by all commercial retail honeys. Many organisations use the Codex Standard for Honey but importing countries may use this standard with their own stipulation that vary in specifications. This work examined the quality of imported honey arriving at the Port of Jizan in Saudi Arabia. The objective of this study was to analyse the imported honey from different regions around the world in order to highlight the variances in the quality of honey by country. In addition, the results of this study will provide guidance to importers as well as competent authorities about breaches and practices in the countries of origin. Recommendations for Good Agricultural Practice (GAP) that incorporate HACCP and rigorous auditing are made.
Materials and Methods
Sample Collection
Our approach utilises the sampling method by Grainger (2000) [18]. Product arriving at the Port of Jizan during 2018 was placed on hold until the final results were obtained. Each consignment was randomly sampled at 2.5% of the volume of shipment. Drums were thoroughly mixed using a paint mixer for five minutes. After allowing two minutes for settling of contents, three samples were removed, one from the top, one from the centre and one from the bottom. Each sample was analysed in triplicate. Any product in bottles within cartons was also sampled at the above rate.
Analytical Procedures
Determination of Pesticides
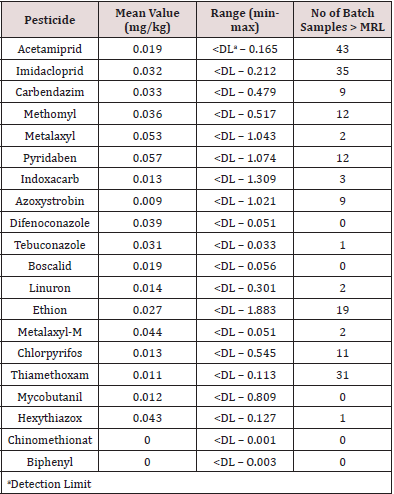
Pesticide analysis was conducted using the procedure by Camino-Sanchez et al (19) as reported in Khatri et al. (20). The pesticides acetamiprid, imidacloprid, carbendazim, methomyl, metalaxyl, pyridaben, indoxacarb, azoxystrobin, difenoconazole, tebuconazole, boscolid, linuron, ethion, metalaxl-m, chlorpyrifos, thiamethoxam, mycobutanil, hexythiazox, chinomethionat and biphenyl were determined by means of liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) using standards obtained from Dr. Ehrenstofer GmbH (Germany).
Extraction Procedure
Accurate sample weights of 10±0.1g were measures and then samples were transferred into a 50ml PTFE tube (extraction kits). To this 10ml acetonitrile was added and shaken vigorously for 1 min. Buffer salt was added. The mixture was then shaken vigorously for 1 min and centrifuged at 10 000 RPM for 10 min. The upper clear solution was transferred into dispersive solid phase extraction tubes (15ml Polyethylene tube) containing 150mg primary secondary amine (PSA) and 900mg anhydrous magnesium sulphate. The tube was capped and the extract was mixed with sorbent and vigorously mixed for 1 min followed by centrifugation at 4000 RPM for 5 min. Two millilitres of the clear extract was transferred into stoppered vials.
Analytical Procedure
The preferred technique for determination of multiresidue methods reported for fruits and vegetables are based mostly on the use of liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS). LC‐MS/MS was performed with an Agilent 1200 series HPLC instrument coupled to an API 3200 Qtrap MS/MS from Applied Biosystems with electrospray ionization interface (ESI) (AB SCIEX, Dublin, CA, USA) and operated under unit mass resolution. The pesticide analysis procedure was conducted as reported in [15] by Sanchez et al (2010). The samples were extracted following the quick, easy, cheap, effective, rugged and safe method known as QuEChERS.
A 20μl sample extract was injected for chromatography into a C18 column ZORBAX Eclipse XDB‐C18 4.6x150mm, 5μm particle size (Agilent, Santa Clara, CA, USA), in which Mobile Phase A contained 5mM ammonium format and Mobile Phase B was methanol. An ESI source was used in the positive mode, with nitrogen as the nebulizer curtain gas. Other gas settings were optimized according to recommendations made by the manufacturer; source temperature was 300 °C, gradient elution programme was 0.3ml/min flow, ion spray potential: 5500 V, de‐cluster potential and collision energy were optimized using a syringe pump by introducing individual pesticide solutions into the MS instrument to allow optimization of the MS/MS conditions.
Identification and Quantification
The selected reaction monitoring (SRM) mode was used in which one transition ion product was used for quantification and the other for confirmation. The identification of a pesticide residue was considered to be confirmed when the retention time of the pesticide matched with that of the pesticide in the pure standard in and the appearance of two product ion transitions that matched the relative intensity criterion under SRM conditions. Once the presence of a pesticide residue was confirmed in an extract, the concentration of the residue was obtained from the appropriate calibration function which corresponds to the matrix‐matched calibration standards. Calibration standard curves were produced by plotting the peak areas for each pesticide versus its concentration with the matrixmatched standard solution and used for the quantification of each pesticide in the sample extract. All sample analyses were conducted in triplicate. The standard curves were linear in the range 0.005- 0.200μg/g with correlation coefficients greater than 0.998. The concentration of the pesticide in the sample extract, Cs (μg/g), was calculated using the following formula:
Cs = Ci x Vtot/Ve x Vf/W
Where:
Cs = sample concentration (μg/g)
Ci = injection concentration (μg/ml)
Vtot = total volume of extraction (ml)
Ve = volume for evaporation (ml)
Vf = final volume (ml)
W = sample weight (g)
Antibiotic Determination
Chloramphenicol testing was achieved using the method provided by Ortelli et al. [10]. LC-MS/MS was utilised to test samples against a chloramphenicol standard from Thermo Fisher Scientific (UK). The AB SCIEX Triple Quad 3500 system enables relatively rapid laboratory performing antibiotic testing and was operated with Turbo V source and Electrospray Ionization (ESI) probe set to 500°C. QuEChERS extracts were diluted 10 times with water to minimize possible matrix effects. Honey samples were diluted with 5 times water and injected directly. LC separation was achieved using a Phenomenex Kinetex Biphenyl 2.6u (50 x 2.1mm) column and a fast gradient of water and acetonitrile with 0.1% formic acid at a flow rate of 0.5 mL/min. An injection volume of 10μl was used.
Determination of 5-Hydroxymethylfurfural
HMF content was measured using method by Winkler [23] as reported in Zapalla et al. [24]. Ten grams of honey were dissolved in 20ml water and transferred to a 50ml volumetric flask. Exactly 2ml of the diluted honey solution and 5.0ml of p-toluidine solution were placed in two separate test tubes; to the first tube 1ml of distilled water was added (this acted as a reference solution); to the second tube, 1ml of 0.5% barbituric acid solution was added (this was the sample solution). The absorbance of the sample was measured against the blank at 550nm was determined using a Varian UVVIS Cary 400 spectrophotometer. For the calibration, a standard solution of 0.300μg of HMF was spectrophotometrically assayed. The quantitative value of HMF was calculated using the proposed formula for the method [25].
Statistical Analyses
Data analysis was performed using SPSS software, version 19.0 (IBM Corporation, Armonk, NY). Descriptive statistics for frequencies and ranges were used to summarise the variables of interest.
Results and discussion
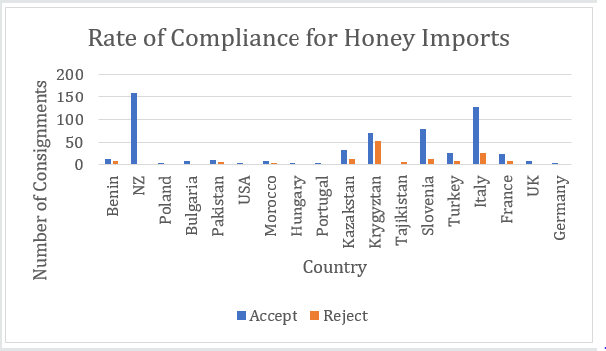
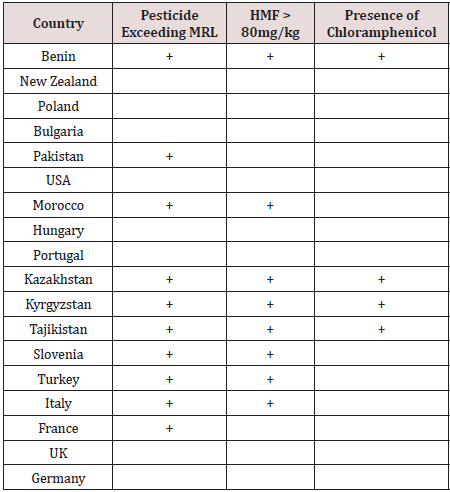
A total of 712 batches of product from 18 countries (Benin, New Zealand, Poland, Bulgaria, Pakistan, USA, Morocco, Hungary, Portugal, Kazakhstan, Kyrgyzstan, Tajikistan, Slovenia, Turkey, Italy, France, UK and Germany) were analysed. Products were rejected based on any one of the following - exceeding the pesticide MRLs or presence of banned pesticides, detection of the antibiotic chloramphenicol or HMF greater than 80mg/kg. Figure 1 shows that 19.9% of product was rejected (n=142) with 80.1% being accepted (n = 570). The countries breaching the limits are shown in Figure 2. These countries were: Benin 8 batches, Pakistan 6 batches, Kazakhstan 12 batches, Kyrgyzstan 52 batches, Tajikistan 6 batches (all being rejected), Slovenia 12 batches, Turkey 9 batches, Italy 27 batches and France 8 batches. The number of accepted batches were 13, 161, 3, 8, 10, 2, 8, 2, 1, 32, 69, 78, 25, 126, 24, 8, and 3 for Benin, New Zealand, Poland, Bulgaria, Pakistan, USA, Morocco, Hungary, Portugal, Kazakhstan, Kyrgyzstan, Slovenia, Turkey, Italy, France, UK and Germany respectively. The number of samples from various batches containing different pesticides is shown in Table 1. There were 192 breaches of MRLs in the 712 batches. One or more of these pesticide residues was present in some of the imported batches. Thus, neonicotinoids coexisted with other pesticides which could increase harmful effects to pollinators and humans. Ethion, acetamiprid, carbendazim and imazalil are banned in Saudi Arabia and their presence is a concern for importers as well as exporting bodies. Table 2 the reasons applied for rejection are provided by country of origin. Benin, Kazakhstan, Kyrgyzstan and Tajikistan had consignments rejected due to the presence of chloramphenicol, exceeding the MRLs authorised for human consumption and levels of HMF greater than 80mg/kg. Batches from Morocco, Slovenia, Turkey and Italy contravened the MRLs as well as the HMF requirements while honey from Pakistan and France breached the MRLs only.
In the study by Mitchell et al. [11], they found neonicotinoids in 75% of the samples, although, concentrations in all cases were below the admissible levels. Many pesticides were present in tandem with others. Despite this fact, evidence from two fairly recent studies [26-27] on the impact of neonicotinoids on human health could warrant re-evaluation of the MRLs towards more stringent levels and control measures, especially, when up-regulation of nicotinic a4b2 Achars receptors in mammalian brains during long-lasting exposure and higher affinity metabolites have been found using imidacloprid. Sub-lethal effects of neonic pesticides on bees have been documented as suppression of the immune system, cognitive ailments, impaired reproductive function, queen survival and poor honing capacity [11]. The level of HMF in honey is an indicator of freshness and quality. It is formed from reducing sugars on heating in the Maillard reaction under acidic conditions. Typically, HMF is absent in honey (or is present in only very small amounts in fresh honeys), while its concentration tends to rise during processing and/or because of storage. HMF has been shown [22, 28] to have negative effects on human health, such as cytotoxicity toward mucous membranes, the skin and the upper respiratory tract, mutagenicity, chromosomal aberrations and carcinogenicity toward humans and animals. The maximum levels of HMF used in international trade is 40mg/kg. However, a level of 80mg/kg is used for tropical honeys and bakers honey.
Chloramphenicol is normally used to control bee brood disease [8]. Both Codex and EU Standards prohibit the use of antibiotics in honey. It is permitted in some countries such as India and Iran [29], Turkey [30] and has also been detected in samples from China that were imported into Canada [31]. Concerns relating to antibiotics include allergic reactions in hypersensitive individuals and disorder of the haemopoietic system, or problems indirectly through induction of resistant strains of bacteria. It is quite clear that the quality of honey provides invaluable information about certain aspects in the supply chain. Measuring pesticide MRLs and antibiotic residues (in this case chloramphenicol) have revealed issues about misuse of neonicotinoids and prohibited pesticides and excessive use beyond internationally recognised standards, several pesticides used in combination as well as some of the countries flaunting the regulations and utilising banned substances. Measuring the HMF level reveals information on further processing which includes heating and also of its age. Bee and other pollinators, the environment and human health are at risk and therefore agricultural authorities are urged to provide appropriate and rigorous training for the use of pesticides, provide a clear understanding between importing and exporting bodies as well as importing country legislation. Furthermore, auditing of facilities with rigid specifications should include HACCP requirements on farm in line with GAP.
Conclusion
Imported honey into Saudi Arabia from eighteen countries was analysed for pesticides, chloramphenicol and HMF. Approximately 20% of imported honey was rejected. Moreover, imported honey from 10 countries breached the MRLs and in some cases, with pesticides that have been banned as well as the presence of chloramphenicol from four countries. HMF was in excess of 80mg/ kg from 8 countries. Routine monitoring programmes for pesticides in honey can assist in the prevention, control and reduction of pollution of the environment and minimise risks to health. However, more rigid approaches to the management of pesticide along the supply chain are necessary. These include training for all individuals concerned with specified objectives, audit schemes on bee farms, assisting bee farmers to reduce the risks of contamination, understanding of legal requirements and specifications in domestic and international trade as well as cooperation between competent authorities and exporting countries. The reduction of pesticide use, in particular neonicotinoids is essential as bee and other pollinators are at high risk. With dwindling bee populations across the globe, the long-term production of honey that is sustainable and safe for human consumption will require agriculture authorities, policy makers and epidemiologists to intervene rapidly as the supply of honey may be threatened.
Read More About Lupine Publishers Journal of Food and Nutrition Please Click on Below Link:
https://lupine-publishers-food-and-nutrition.blogspot.com/


No comments:
Post a Comment
Note: only a member of this blog may post a comment.