Lupine Publishers| Journal of Drug Designing & Intellectual Properties
Abstract
Alginate is a natural polysaccharide that is widely used as a component of pharmaceuticals and in food industry. Alginate particles can be used for encapsulation of substances with the necessity of prolonged release. They can also provide appropriate microenvironment for cells. Here the methods of the synthesis of alginate beads, micro- and nanoparticles are reviewed with special attention to the calcium alginate ones. The results from publications that did not deal with alginate particles but, to our opinion, could be applied in this field are also included in order to give an outline for possible future research. The suggested applications of the particles are mentioned as well. The two main methods for the synthesis of calcium alginate particles are internal and external gelation, but the external gelation techniques can be themselves subdivided into several subtypes. Currently, a technique being able to produce alginate nanoparticles with any desirable size does not exist. We analyze the possibilities of employing aerosolization method for this purpose. The potentials to overcome the problem of burst release of the encapsulated substances by means of cyclodextrin inclusion complexes and employing additional crosslinking agents are also discussed. The clinical application of alginate nanoparticles is still limited because of the burst release of encapsulated drugs and the poor size control of the particles formed. Further research must concentrate on overcoming these problems and on topical application of alginate particles without entering bloodstream rather than on investigation of model drug release in vitro without taking the above-mentioned problems into account.
Keywords: Calcium alginate; Alginate beads; Alginate microparticles; Alginate nanoparticles; Drug encapsulation; Particle size control; Aerosol
Introduction
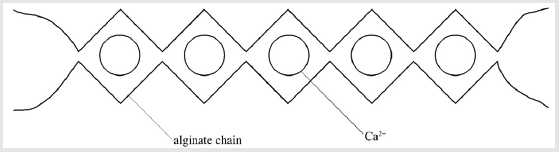
Alginates are polysaccharides. The commercially available ones come from brown algae. They are linear copolymers of (1→4)-linked units of β-D-mannuronic acid and α-L-guluronic acid. The molar ratio between them and their distribution along the commercial polymer depend on the algal source, its location, age, collection season and extraction technique. Guluronic acid residues can form so-called egg-box complexes with calcium ions or some other divalent metal cations leading to gel formation (Figure 1). The name ‘egg-box’ is used because, if depicted schematically, the cations look like eggs situated inside puckered boxes formed by four guluronic acid residues of two superimposed chains. Mannuronic acid residues have much less affinity to metal ions [1]. Barium ions have more affinity to alginate than the calcium ones. If reacted with calcium alginate at lower concentrations, they create new gelling junctions. At higher concentrations, barium ions also displace calcium ions from existing junctions [2].
To extract alginate, algae are usually washed with organic solvents and water, dried and milled. After acid pretreatment, alginate is solubilized with Na2CO3 . The crude extract is concentrated, dialyzed against water and then freeze-dried or precipitated with ethanol. Acidification or treatment with Ca2+ can be used instead. Brown algae are abundant in nature; however, the possibility of cultivating them exists as well [1]. The viscosity of aqueous sodium alginate solution rapidly increases with its concentration. For example, the addition of 10% of alginate to water leads to a ~100-fold increase in viscosity. But the poly electrolyte nature of alginate has little effect on its hydration, and in the above example less than 3.7% of the water molecules present in solution is involved in alginate hydration. Such a large viscosity increase is determined by the polysaccharide network, with large bulk-like water pools present between the polysaccharide chains [3].
Sodium alginate may act as a mucoadhesive polymer. A comparative study of adhesion between buccoadhesive compacts and pig buccal mucosa or sodium alginate solution revealed that the results were of similar performance [4]. Sodium alginate was proposed as a mucoadhesive component of a nasal gel [5] or in buccal patches containing salbutamol sulfate [6]. Sodium alginate conferred in situ gelling mucoadhesive properties and retarded drug release from liquid rectal suppositories. These suppositories were successfully tested on Guinea pigs to alleviate symptoms of histamine-induced bronchospasm [7]. Sodium alginate was also evaluated as an excipient in salbutamol sulfate sublingual films [8] and tablets [9]. However, drug release was found to be too slow in the films [8] or too rapid in tablets [9]. Only salbutamol sulfate tablets formulated from granules containing mastic and sodium alginate excelled commercial tablets in the terms of drug release when tested on rabbits [10]. In combination with hydroxy propyl methyl cellulose and propylene glycol sodium alginate was used in the formulation of terbutaline sulfate sublingual films [11]. Sodium alginate could be also used as a component of plugs for water-soluble parts of crosslinked gelatin capsules containing pellets with encapsulated salbutamol sulfate. The plug absorbed the surrounding fluid, and began to release the drug through the swollen matrix and was finally ejected out of the capsule by erosion of the material. The usability of the system was shown on rabbits [12].
Chitosan-alginate complex was proposed as an excipient for orodispersible tablets, and their disintegration time was so short that it was even referred to as a ‘super dis-integrant’ [13]. One optimized formulation containing the excipient for 5-fluorouracil tablets, suitable for trans buccal and rectal drug delivery, contained this chitosan-alginate complex along with the same components un-complexed in order to avoid burst release and to improve the mucoadhesive properties [14].
Sodium alginate itself also has a therapeutic effect. When admixed to foods for diabetic human patients, it decreased gastric emptying rate and rises glucose in blood, serum insulin and plasma C-peptide levels [15]. Orally administered, sodium alginate significantly alleviated small intestinal enteritis in rats, caused by treatment with the anti-inflammatory drug indomethacin, and this relief seemed to be independent of the sodium alginate viscosity administered [16]. Oral disposal of sodium alginate to rats with colitis led to a significant reduction of colonic damage, decreased lesion formation [17,18] and inhibited mucosal injury [17]. Orally administered alginate oligosaccharide obtained from hydrolysis of sodium alginate by Bacillus subtilis improved histopathological and biochemical parameters of mice having ovalbumin-induced asthma in a dose-dependent manner [19]. Rats fed with sodium alginate drank more water, and their urine volume and pH rose sharply. In contrast, calcium alginate caused very little changes in the same parameters [20].
Commercially available calcium alginate swabs were used for sampling nasal flora for subsequent DNA extraction [21]. The mucoid exopolysaccharide produced by the pathogenic bacterium Pseudomonas aeruginosa is alginate, but it has low immunogenicity if it is not conjugated with a carrier protein. Even in the form of conjugate it is non-toxic if administered intraperitoneally to mice or guinea pigs and non-pyrogenic if administered intravenously to rabbits [22]. When alginate beads with encapsulated tumor cells were implanted to mice, a process of angiogenesis was observed in the implants zone. The beads were prepared from commercially available alginate [23]. It may be considered as a further proof of alginate biocompatibility.
Sodium alginate was also shown to be beneficial for agriculture. Being administered as dietary supplement to the white shrimp Litopenaeus vannamei, it acted as an immunostimulant and improved its resistance against the attack of Vibrio alginolyticus bacterium [24]. Sodium alginate digested with alginate lyase promoted root elongation of rice, carrot [25], lettuce [26] and barley plants [27,28] even under hypoxic conditions [28]. It was hypothesized that digested alginate might initiate some signal transduction pathway [27,28]. Under hypoxic conditions digested alginate also caused enhancement of the activity of the enzymes regenerating NAD+ [28].
Alginate oligomers promoted the germination of unhulled rice and Komatsuna seeds as well as tobacco callus differentiation. The mixture of oligomers was assumed to contain so-called oligosaccharine, an oligosaccharide inducing unusual proliferation and/or differentiation of plant cells. There are several kinds of oligosaccharines. They act as a chemical signal for the stimulation of hormone synthesis [29]. The promoting effect of alginate oligosaccharides on root formation and growth in rice was mediated by endogenous indole-3-acetic acid [30]. But proliferation of the microalga Chlamidononas reinhardtii was repressed by the same oligomers [29]. It should be noted that only digested sodium alginate shows this effect. And the possibility of alginate degradation by the lyases of soil bacteria is assumed [28]. Therefore, it is speculated that these active substances can be formed from alginate under natural conditions. Another notable advantage of alginate is its ability to bind micronutrients. Some important Mn, Cu, Zn and Mo fertilizers are MnCl2 , CuSO4 ·5H2O, ZnCO3 and Na2 MoO4 ·2H2O, respectively [31]. Manganese can be complexed with alginate by addition of MnCl2 to the gelling solution of CaCl2 or BaCl2 , and slow release of manganese ions from the beads into physiological saline has been reported [32] because the affinity of alginate to Ca2+ is higher than to Mn2+ [33]. The beads containing Ba and Mn could be used for manganeseenhanced magnetic resonance imaging and were tested on rats [32]. The use of CuSO4 [34] or CuCl2 [35] as a gelling solution led to copper alginate hydrogel being able to release copper ions into simulated body fluid [34] or into phosphate buffer (pH 6) [35] in a prolonged manner. The use of basic zinc carbonate for zinc alginate hydrogel formation using internal gelation method has also been reported. The hydrogel was active against E. coli [36]. Molybdenum can be adsorbed by preformed calcium alginate beads preferably in the form of H2[MoO4] or [Mo(H2O)6]3+ at pH 2 and released back up to 50% into 0.1M HCl. If radioactive molybdenum is used, the method is suitable for radiotherapy [37]. Copper ions from CuCl2 may also be adsorbed onto preformed calcium alginate beads [38]. Copper alginate shows activity against Staphylococcus aureus, Staphylococcus epidermidis, Staphylococcus pyogenes and E. coli [34].
The biodegradability of unmodified alginate particles, their ability to bind micronutrients and the beneficial effect of alginate make them promising carriers of agrochemicals. This is especially important because in a recent review [39] the authors expressed great concerns regarding the use of nanoparticles in agriculture because of the negative impact of metal and oxide nanoparticles on soil microorganisms, earthworms and even on cultivated plants.
Alginate beads can also have industrial applications:
• Wastewater treatment. The beads with encapsulated horseradish peroxidase could be reused up to 3 times, although the encapsulation decreased enzyme activity in comparison with the free enzyme [40]. In another report, the efficiency of phenol removal by encapsulated horseradish peroxidase was demonstrated by reducing to half the initial phenol quantity after only 5 reaction cycles [41].
• Food industry. Xylanase immobilized in alginate beads may be used for fruit juice clarification [42]. Lactobacillus helveticus and Streptococcus thermophilus immobilized in alginate beads were intended for use as lactic starters in milk fermentation [43]. Alginate particles with encapsulated healthy nutrients can also be used as components of functional foods.
• Enzyme production by cells immobilized in alginate beads. A good example is glucoamylase [44]. In this case fungus Thermomucor indicae-seudaticae was immobilized in alginate beads, and cane molasses was used as a cheap medium [44]. In all these applications alginate is exploited because of its natural origin, i.e. it cannot be a harmful admixture if separated incompletely. Beads (and not nanoparticles) are chosen because they can be easily separated by sedimentation. As could be seen above, not only enzymes themselves but also enzyme-producing bacteria can be immobilized in alginate beads.
In contrast to metal nanoparticles, alginate particles can be modified either before or after their synthesis. In the former case, bulk alginate is modified and then used to prepare the particles. This way is usually preferable from the two options because it avoids the leak or destruction of encapsulated substance during the modification. The chemical modification of alginate is reviewed widely in [45].
Alginate microparticles and nanoparticles are usually used to encapsulate and carry various substances, and the goal of many studies is to achieve sustained release of them. It should be noted, however, that the results of experiments dealing with the release of poorly water-soluble drugs might often be misinterpreted, because the drug not found in the solution is assumed to remain encapsulated in the particles. However, it may decompose after the release or simply precipitate out. Supersaturated solutions with varying extent of supersaturation can also be formed, making the results irreproducible. Special care must be taken in the case of putting the particles into a dialysis bag, because the films act as an additional diffusion barrier. If centrifugation is used for separation of the medium with the released substance from the nanoparticles, the pressure generated during the process can disturb the equilibrium. It can also make difficult resuspending the nanoparticles in the fresh portion of medium for further incubation [46].
For consistency, throughout this review we will use the following terms (even if different names for them were used in the respective publications):
• Encapsulation efficiency: the percentage of the substance that was encapsulated (i.e. not lost).
• Loading efficiency (expressed in percent): the ratio of the weight of the successfully encapsulated substance regarding to the total weight of the particle. Some authors calculate loading efficiency using different formulae, but we will give their values without a special discussion. We have rounded encapsulation and loading efficiencies as well as zeta potentials to the nearest integer values.
• Nanoparticles are considered smaller than 1µm. Microparticles have size from 1 to 1000µm. Beads have size in a millimeter range. We have rounded bead size to the first decimal place.
Also, some authors term their particles ‘microcapsules’. However, we will use this term only if they have demonstrated or at least assumed the presence of a liquid core in their particles. In other cases, we will refer them to as microparticles.
Alginate-chitosan particles
Since alginate is a polyanion and chitosan is a polycation, they can form a polyelectrolyte complex upon mixing, provided both of them are charged, i.e. at suitable pH. This mixture can spontaneously form particles. The pKa of alginate carboxyl group is close to 5, and that of the ammonium group of chitosan is about 6.2 [1].
Alginate-chitosan nanoparticles were prepared by dropwise addition of a chitosan solution containing glutathione into an alginate solution at pH 4, under stirring. If prepared at 0.75 alginate: chitosan ratio, the formed nanoparticles with encapsulated glutathione had the following characteristics: size 361nm, polydispersity index 0.33, zeta potential 27mV [47,48]. At 1.5 alginate: chitosan ratio, the values were 212nm, 0.4 and 23mV, respectively, although the storage stability decreased, making these nanoparticles less suitable for application. In the same way, the pH increase from 5.0 to 6.5 and further caused aggregation [48]. The encapsulation efficiency was 27% [47] or 80% at ratio 0.75 and fell to 1% at ratio 1.5 [48]. The respective investigations were aimed to achieve the synthesis of mucoadhesive nanoparticles with an encapsulated NO donor needed for treat important diseases because of the multifaceted role of NO in vivo. Therefore, encapsulated glutathione was nitrosated inside the nanoparticles by adding sodium nitrite to the solution. S-nitrosoglutathione decomposition at 400µM was delayed by its encapsulation in the nanoparticles. At 18µM encapsulated S-nitroso glutathione was not cytotoxic to cultured Chinese hamster lung fibroblast cells (V79), whereas free S-nitroso glutathione was slightly cytotoxic at the same concentration. This assay could enable the use of these anti microbial nanoparticles in pharmaceutical applications such as wound healing without severe side effects [47,48].
Later, the same technique was used by the same group to encapsulate mercaptosuccinic acid and nitrosate it inside the particles. In this case, the hydrodynamic size of the nanoparticles was ~750nm. The encapsulation efficiency was 89%. Burst release of NO in aqueous solution was followed for 4 hours, although the release in the normal mode continued for 6 hours more. These nanoparticles were assayed for topical application for bovine mastitis. The minimal inhibitory concentration of the nanoparticles for Staphylococcus aureus determined in vitro was 125-250µg/ml. The number of colony forming units was 10-fold and 1000-fold lower after bacteria were incubated with nitrosated nanoparticles at 500µg/ml for 4 and 7 hours, respectively, compared with bacteria growth in the presence of empty nanoparticles at the same concentration and time. The CFU drastically decreased further upon the addition of a second dose of nitrosated nanoparticles. For E. coli the minimal inhibitory concentration exceeded 2000µg/ml, i.e. these nanoparticles were inefficient against this bacterium. The 50% cytotoxicity concentration of the nanoparticles for cultured HEp-2 cells was 640µg/ml. Chitosan nanoparticles without alginate at the same concentrations of the acid released more NO at higher rates. Nevertheless, it was concluded that NO-releasing nanoparticles might be used to combat bacteria for treating and preventing bovine mastitis [49].
Spherical alginate-chitosan beads with encapsulated lemongrass oil having size of 1.8-2.1mm displayed significant antibacterial and antioxidant activity. For unencapsulated oil the same activity was observed only at higher concentration. This beneficial action was attributed to the strong interaction between chitosan and the oil. This kind of beads has potential applications as a greener agent for medical purposes [50].
The following advantages of alginate-chitosan particles can be underlined:
• Chitosan can enhance drug bioavailability by its capacity of infiltration into the mucus layer of the small intestine with subsequent opening of tight junctions of epithelial cells [51].
• Unlike calcium alginate, alginate-chitosan polyelectrolyte complex cannot be disintegrated by chelatoring agents. Their main disadvantage could be the necessity to use an acidic solution of chitosan because of its insolubility at neutral pH.
Preparation of alginate particles without employing gelation
Now we describe the techniques for preparation of particles from bulk sodium alginate or its solution as well as spontaneous formation of particles of modified alginic acid in water. The resultant particles are usually intended to be ready to use. However, dry sodium alginate particles can be later treated with CaCl2 solution in order to convert them to calcium alginate particles.
There exists a patented technique for producing alginate, cellulose, starch or collagen particles from bulk substances by ball milling with the possibility to control particle size from 100nm to 50µm. The resultant nanoparticles containing therapeutic proteins have shown efficacy in treating solid tumors, single dose vaccination, and oral delivery. For instance, tumor-bearing mice that received these nanoparticles containing Texas red and cisplatin showed significant tumor size diminishing. If the same nanoparticles were coupled to dendritic cell-binding peptide and contained encapsulated pneumococcal surface protein A, together with an adjuvant, they were effective to combat the bacterial load of the mice that was reduced (in the terms of infected tissue volume) after exposition to nanoparticles. The nanoparticles produced by the same milling technique were also used to induce passive immunity against anthrax toxin in mice by means of oral delivery of monoclonal antibodies developed versus anthrax toxin [52].
Another technique consists in dropwise addition of pure ethanol or acetone to 1% sodium alginate in water containing drug solution in dimethyl formamide. Mixing [53] and cooling down to 3-5 °C is needed during the process. At low mixing speed aggregation was observed [54]. These microparticles can be separated by filtration, washed with the same solvent and dried on air, in a heating oven [53] or in a desiccator [54]. Using nitrofurazone as an example of an encapsulated drug, the loading efficiency and yield of microparticles decreased as the particle size increased from 5 to 30μm with ethanol dripping rate increasing. The presence of 0.1% ammonia [54] (pH 8-9) and of a surfactant was needed in order to avoid particle aggregation in the case of nitrofurazone. Other drugs, viz. acridone, tetracycline, dibazole and metronidazole were encapsulated in the same way (but without ammonia), although the encapsulation conditions needed to be optimized for every drug separately. The yield varied from 31% for metronidazole to 77.5% for tetracycline, and the loading efficiency varied from 2% for metronidazole to 43% for nitrofurazone [53]. In a later publication, the same group reported that in the case of nitrofurazone the yield of microparticles was 81% with a loading efficiency of 34%. Spray drying instead of filtration was recommended to increase the yield [54]. The stability of nitrofurazone-loaded microparticles resuspended in water was reported to increase with pH [53]. At 1% and 2% of particles (nitrofurazone concentration was 0.34% and 0.68%, respectively) these solutions were more active against E. coli, P. aeruginosa, P. vulgaris, S. aureus and B. subtilis than aqueous nitrofurazone solution having drug concentration less than 0.02% because of its insolubility. In the case of Candida albicans the same solutions of microparticles excelled in antifungal activity nitrofurazone solutions in DMSO with the concentrations of 1% and 2% [54]. The ability of the particles to form stable suspensions and to enhance drug solubility in water broadens the field of drug application [53,54]. The encapsulated drugs are expected to be more stable under ambient conditions [53].
Spray drying the sodium alginate solution containing the payload (caffeine-loaded peptidic nanoparticles) yielded microparticles having size of about 4μm. The crosslinking with CaCl2 solution increased their mean size to 7.4µm but decreased their shrinkage and slowed down the release of caffeine into simulated gastric fluids. The particles are potentially bioactive because of the presence of antioxidant peptides [55]. Spray drying the solution containing sodium alginate, pectin and gentamicin sulfate at inlet temperature of 90 ºC was used for wound dressing preparation. The volume diameter at the 50th percentile (spanning from 310 to 1003nm for various samples), the width of particle size distribution, water content and drug release rate increased with nozzle spray mesh diameter and with feed solution concentration at constant ratio of the components. Flowability of the powders, the adhesive strength of the gel formed from them in contact with simulated wound fluid as well as its activity at 0.25mg/ml of gentamicin sulfate against Staphylococcus aureus and Pseudomonas aeruginosa showed the opposite tendency. Antimicrobial activity was expressed as the diameter of the zones of clearance around the two samples spotted on agar plates with the spread bacterial culture after incubation for 24 hours. For Staphylococcus aureus the activity of two samples was also tested in culture medium after 3, 6, 9 and 12 days of incubation. The particles were mainly spherical, but other shapes appeared when either feed concentration or mesh nozzle increased, and further increase led to large collapsed particles. All the particles were composed of smaller aggregated particles. The encapsulated drug was being released in simulated wound fluid in Franz-type diffusion cells for up to 5 days. Loading efficiency was around 24-27% with an encapsulation efficiency between 70 and 83%, and for all the samples initial burst release was observed. At 40 °C and 75% relative humidity drug content was preserved for 6 months with only slight increase in water content. Swelling rate in contact with simulated wound fluid depended on particle size. The yield increased with feed solution concentration but decreased with nozzle spray mesh diameter. The nanoparticulate powder may be used as a self-consistent formulation having great potential application in the treatment of both acute and chronic infected wounds [56].
Low molecular weight alginic acid prepared by acid hydrolysis of sodium alginate formed nanoparticles itself (without calcium ions) when its hydroxyl groups were functionalized with oleoyl residues. The nanoparticles were loaded with vitamin D3 by addition of its solution to the reconstituted solution of vacuumdried nanoparticles. Loading efficiency increased with the vitamin concentration from 0.3 to 0.9%, but the encapsulation efficiency also decreased from 68% to 46%. Mean nanoparticle hydrodynamic diameter also decreased from 559nm to 305nm, and particle formation rate was sped up when substitution degree increased. An unimodal particle size distribution was revealed. In simulated gastric fluid they retained spherical shape and released ~40% of the encapsulated vitamin for 3 hours. But in simulated intestinal fluid they became irregularly shaped, their hydrodynamic diameter was 757nm and burst release of 40% of the vitamin occurred, with 60% of the vitamin released after 7 hours. The nanoparticles can be used as oral carriers for liposoluble nutraceuticals [57]. The great disadvantage of sodium alginate particles is the very limited possibility of prolonged drug release because of sodium alginate solubility leading to fast disintegration of the particles. The particles of modified alginic acid offer convenient manipulation, but the need of its prior chemical functionalization limits the applicability of the technique for non-specialized, e.g. biomedical laboratories.
Production of Alginate Particles Using Other Particles as Cores
In this method, sodium alginate is physically adsorbed or covalently linked to the surface of other particles. The particles can also be formed already capped with alginate. In some cases subsequent gelation with CaCl2 is carried out. For CaCO3 particles there is a possibility of their generation simultaneously with alginate gelation.
Dropwise addition of chitosan nanoparticles with encapsulated bovine serum albumin modified with rhodamine isothiocyanate to sodium alginate solution at controlled pH yielded negatively charged nanoparticles having hydrodynamic diameter of several hundred nanometers depending on the solution composition. The highest diameters were registered in water. The nanoparticles successfully delivered the protein into cultured cells, with the localization depending on cell type. Significant increase in peroxide production by HCEC cells was observed at 300 and 600µg/ml of empty nanoparticles after exposure for 4 hours. However, there was almost no superoxide production after either 4 or 24 hours of exposure. The metabolic activity of LN229 and MCF-7 cells remained unchanged for up to 72 hours of incubation with the empty nanoparticles. But MDA-MB-231 and HCEC cells displayed significantly decreased metabolic activity at nanoparticle concentration above 180µg/ml after 72 hours of exposure, but not after 24 hours. Similar survival decrease at these concentrations of nanoparticles was observed for A549 cells. Dose dependencies acquired after 24 or 72 hours of exposure were almost the same. Survival of HT29 and CaCO2 cells was significantly increased only after exposure to 600µg/ml of the nanoparticles for 72 hours. The nanoparticles have potential applicability as nanocarriers in cancer therapy [58]. A similar technique was used for enoxaparin encapsulation. The proposal was to evaluate nanoparticles loaded with this low molecular weight heparin for its oral delivery, controlled and prolonged release in order to improve patient compliance. In this, chitosan nanoparticles were covered with sodium alginate (applied in phosphate buffer) and treated with CaCl2 . Parameters of the optimized formulation were as follows: average size 335nm, spherical, polydispersity index 0.37, zeta potential –31mV, encapsulation efficiency >70%, drug release in simulated gastric fluid for 2 hours 2%, in simulated intestinal fluid for 14 hours ~60%. Degradation and erosion of nanoparticles was identified as a possible drug release mechanism. The pharmacokinetic parameters of the drug given orally to fasted rats through cannula in a dose of 50mg/kg body weight were improved. Nevertheless, those of intravenously administered free enoxaparin at 1mg/kg were better. 75% of the encapsulated drug applied at 2mg/ml reached across the intestine to the serosal fluid for 90 minutes, as shown in vitro by means of everted intestinal sac model. 900 IU of orally administered encapsulated drug reduced thrombus formation by 59% compared with buffer. Significant uptake of the nanoparticles by the intestinal mucosa for 1 hour was shown by administration of nanoparticles loaded with fluorescein isothiocyanate instead of the drug through gastric cannula to fasted rats. Therefore, the nanoparticles proved their utility as oral delivery vehicle for enoxaparin. Such a vehicle is a foremost requirement for non-invasive and non-hospitalized treatment of vascular disorders (deep vein thrombosis, pulmonary embolism and venous thromboembolism). But subcutaneously injected free drug was even better [51].
Read More About Lupine Publishers Journal of Drug Designing & Intellectual Properties Please Click on Below Link: https://lupine-publishers-drug-designing.blogspot.com/

No comments:
Post a Comment
Note: only a member of this blog may post a comment.