Lupine Publishers | Journal of Veterinary Science
Hens were immunized with a macerate of goat and sheep placentomes. Immunizations were repeated every 15 days. Eggs were collected over a period of 03 immunizations, IgY isolated, and its concentration measured. After 45 days of the start of immunization, chickens were euthanized, their spleens isolated and fragmented for total RNA extraction by Trizol method. Performed the synthesis of cDNA, was amplified by PCR the fragments of immunoglobulin G avian variable light chain (VL) and heavy chain (VH). Fragments of VL and VH were used to constitute the final product by recombinant PCR-overlap process. The results present the feasibility of building a library of scFv anti goats and sheep placenta opening the possibility of manufacture diagnostic kits for various physiological situations of placental trophoblast cells belonging to these species.
Introduction
In ruminant animals, possessing synepthelichorial placenta (Igwebuike [20]; Pinto et al. [21]), the activity of trophoblast cells, especially the trophoblast giant cells, are related to the process of implantation of the conceptus and training of the placentome Pinto et al. [21]; Ealyet al. [22] and directly related to the recognition of the fetus by the maternal organism Shi et al. [23]; Roberts, [24]. This cellular activity induces the production and secretion of various specific molecular structures that are temporarily expressed during pregnancy Miguez et al. [25]; Machado et al. [26], but not all are known or have been isolated. Detection of these antigens or chemical markers is of great importance for breeding ruminant animals and especially for a greater understanding of the physiology and gene expression of trophoblastcells synepthelichorial placenta Roberts, [24]; Roberts et al. [27].
It is presented in this context, this work has as objective aconstruction of gene libraries of single chain variable fragments (scFv) antibody chicken (Gallus gallusdomesticus) placentome anti-ruminant animals and also to contribute to the creation of new knowledge on trophoblastcells and their effects on reproduction in animals synepthelichorial. These gene libraries will be the basis for future work, we will focus on the expression of recombinant biomolecules, which can be selected by affinity for the specific trophoblast cells in goats and sheep placentomes molecular markers.
Materials and Methods
Immunization of animals and collection of samples
Used in this study were macerated placentomas of female goats and sheep consistent with the first third of gestational development. The immunization was performed via the deep pectoral muscles at three Lhomann brown laying hens of 28 weeks old. Immunizations took place every fortnight, the first immunization was performed with the filtrate 200 mg macerate placentome goats and sheep (GSP - Goat and Sheep Placentomes) plus complete Freud’s adjuvant, 1:1. Over two immunizations were performed with the use of incomplete Freud’s adjuvant, under the same conditions for the first immunization. In the process of construction of the gene library of goat and sheep placenta antiscFv mixture of macerated placentome of female goats and sheep was used, this procedure was done this way because of the great phylogenetic closeness existing between these two species. Fifteen days after the third and final immunization, totaling a trial period of 45 days, all chickens were anesthetized using a 5:1 ratio of Ketamine (100mg/ml or 50mg/kg)and Xylazine (20mg/ml or 10mg/kg ) following the animals were euthanized by ensanguinação. The spleens of these animals were isolated, identified, and stored at -20 °C for future total RNA extraction. During the immunization eggs were collected and separated to the extraction of Y (IgY) immunoglobulins performed by using the commercial kit EGGstract ®IgY Purification System (Promega Corporation, Madison, USA), with subsequent measurement of the concentration by the method of Bradforf Bradford[28] and purification of IgY by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate (SDS-PAGE).Extraction and isolation of total RNA
Three fragments of spleen, 50mg, referring to the three chickens were used for extraction of total RNA by methodology TRIZOL reagent (Invitrogen, USA). The quantification of the concentration was carried out in a spectrophotometer (Power Wave NS2 BioTek Instruments, Inc.) at a wavelength of 260nm.Construction of cDNA molecules and the light chain (VL) and heavy chain (VH)
The cDNA of the immunoglobulin G (IgG) was synthesized by using kit SuperScriptTM III Reverse Transcriptase (Invitrogen, USA) and dNTP Set (Fermentas Life Sciences, Maryland, USA), according to the manufacturer’s instructions. Primers used for amplification of the variable regions VL and VH were prepared by BarbasIII et al. [29].VL regions of the immunoglobulins were amplified using the sense primer CSCVK-F (5’-GTG GCC CAG GCG GCC CTG ACT CAG CCG TCC TCG GTG TC-3’); and antisense CKJo-B (5’-GGA AGA TCT AGA GGA ACC CTG TAG GAC GGT CAG G -3’). For amplification of the VH region, we used the sense primer CSCVHo-F (5’-GGT CAG AGA TCC TCT TCC TCT GTG GCC TTG ACG GAC GAG -3’) and antisense CSCG-B (5’-GCC CTG GGC CTG GCC ACT AGT GGA GAT GGA GAC GGT GAC TTC CC-3’) (Eurofins MWG Operon, Alabama, USA). The cDNA samples were pretreated with RNase A 5U/μl (Thermo Fisher SCIENTIFIC, USA), and the PCR reactions were designed to 50ng of each sample Barbas III et al. [29]. Considering the final volume of 50μl per amplification reaction, this was performed using 5U/ μl Taq DNA polymerase (Fermentas LIFE SCIENCES, Maryland, USA); 1 mM MgCl2; 2 mMdNTP Mix (Fermentas LIFE SCIENCES, Maryland, USA), 10x Taq buffer with KCl (100 mMTris-HCl pH 8.80, 500 mMKCl/Fermentas Life Sciences, Maryland, USA), 30pmol/ μl of sense and antisense primer and free water RNA and DNA in order to complete the amplification reaction 50μl. The buildings of the fragments VL and VH were made separately and each using their specific primers.The programming of the recommended thermocycler followed by Barbas III et al. [29] beginning with an incubation at 94°C for 2 minutes. Compounds followed by 30 cycles by denaturing at 94°C for 02 minutes, annealing at 56°C for 15 seconds and extension at 72°C for 90 seconds, and the completion of the reaction cycle at 72°C for 10 minutes was used. The control reaction was performed by electrophoresis in 1% agarose gel and the result was further documented by using photodocumentator.
Construction of single-chain variable fragments (scFv) by overlap-PCR
The bands of fragments VL and VH were extracted from the agarose gel and purified separately with the aid of MinElute Gel Extraction Kit (QIAGEN). Following extraction of these products were further purified by QIAquick PCR Purification Kit (QIAGEN) and stored at -20°C. The Overlap-PCR is define the connection (or overlap) between fragments of the chain light (VL) and heavy (VH), constructed by forming a flexible peptide linker (VL-linker-VH) comprising the complementary tails of the connection between the sense primer (CSCVHo-F) heavy chain and anti- sense primer light chain (CKJo-B) used for amplification of fragments. The primers for the construction of scFv molecule of this study followed the description Barbas III et al. [29]: sense primer CSC-F (5’-GAG GAGGAGGAGGAGGAGGTG GCC CAG GCG GCC CTG ACT CAG -3’) and antisense primer CSC-B (5’-GAG GAGGAGGAGGAGGAGGAG CTG GCC GGC CTG GCC ACT AGT GGA GG-3’) (Eurofins MWG Operon, Alabama, USA). The final product was characterized Overlap-PCR with approximately 750 base pairs (bp).The Overlap-PCR reactions were drawn for each sample 50ng of fragment VL and VH Barbas III et al. [29]. The PCR fragments overlap VL and V H has been designed to use 5U/μl Taq DNA polymerase (Fermentas LIFE SCIENCES, Maryland, USA) 1 mM MgCl2, 2 mMdNTP Mix (Fermentas LIFE SCIENCES, Maryland, USA), 10XTaq buffer with KCl (100 mMTris-HCl pH 8.80, 500 mMKCl/Fermentas LIFE SCIENCES, Maryland, USA), 30pmol/μl of sense and antisense primer and free water RNA and DNA in order to complete the reaction 50μl Overlap-PCR.
The thermocycler was programmed for Overlap PCR reaction so that the activation reaction was carried out at 94°C for 60 seconds, following 30 cycles were performed 03 times with each cycle, the first time 2 minutes 94°C, the second at 56°C for 60 seconds and a third at 72°C for 90 seconds, and the completion of reaction was conducted by one cycle at 72°C for 10 minutes. The control reaction was performed by electrophoresis on a 1% agarose gel and the results were documented through the use of photodocumentator.
Statistical Analysis
To compare the means of IgY the mixture of macerated GSPTukey’s test at 5% significance level was applied, using the statistical program ASSISTAT, version 7.6 beta/2011.Results
Measurement of immunization
Measuring the success of immunization with GSP mixture was performed by assessing the concentration (mg/mL) of purified IgY from the yolks of immunized hens. Immunization with GSP solution showed average concentrations of 2.89±0.12, 3.90±0.15 and 4.24±0.21 mg/mL, respectively for the 1st, 2nd and 3rd immunization. Immunizations performed with the mixture of macerated GSP showed the linear regression equation y = 0.195 x + 7.74 and a coefficient of determination (R2) equal to 0.9916 (99.16%), indicating an excellent fit for the relationship between immunizations and production of IgY performed, ie more than 99% of the production of IgY was established directly by increasing the number of immunizations with a mixture GSP.
Figure 1: Purification results of immunoglobulin Y (IgY) anti-goat and sheep placentames in different stages of immunization.
Polyacrylamide 6% acrilamide gel electrophoresis.MPM, molecular weight marker (kDa).
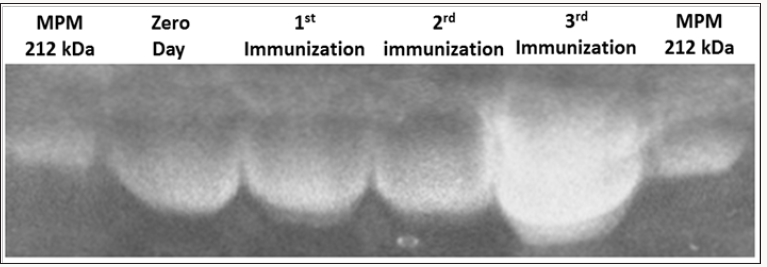
In the purification of IgY by electrophoresis on 6%
polyacrylamide (SDS-PAGE) gel was identified IgY approximately
212 kDa (Figure 1). We can observe an increase in the thickness of
the band related to immunoglobulins, thus confirming higher levels
of concentration of IgY, which corroborates with the increased
concentration of IgY.
Statistical Analysis
The data in (Table 1) corroborates the mixture homogenized with the GSP, in the course of three immunizations promoted increased immunogenicity and consequent increase in the production of IgY as a response. This analysis was provided by the use of the Tukey test at a significance level of 5%.
Table 1: Values followed by the same letter do not differ
statistically among themselves. We have used the Tukey test at
5% significance level. (MSD = minimum significant difference;
GM = general average; CV% = coefficient of variation in %).
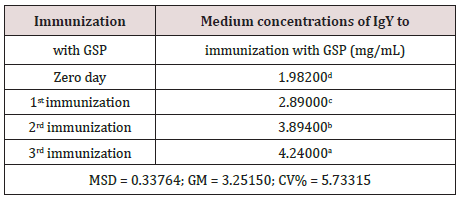

Figure 2: Amplification results of the light chain (VL) and heavy chain (VH) scFv fragments of chicken Immunoglobulin G
(IgG), anti-goat and sheep placentome 1% agarose gel electrophoresis.
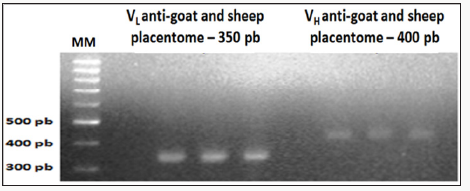

Amplification product of the cDNA of light chain (VL) and heavy chain (VH) scFv chicken immunoglobulin G
Referring to immunization with the macerated GSP after running on 1% agarose gel, the image was identified the presence of VL bands with 350pb and VH bands with 400bp (Figure 2).Product overlapping sequences of scFv fragments by Overlap-PCR
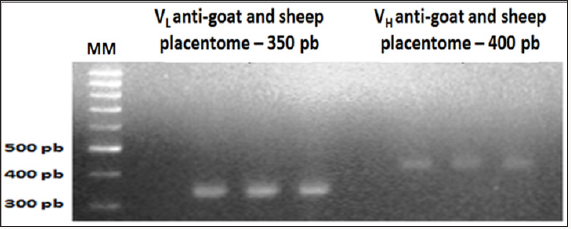
Also referring to mixing with the mash GSP, after Overlap PCR reaction, the formation of fragments with approximately 750pb has been identified as a result of overlapping fragments and linearization VL and VH.Discussion
Figure 3: Overlap results of the light chain (VL) and heavy
chain (VH) scFv fragments of chicken immunoglobulin G
(IgG) anti-goat and sheep placentome (GSP). 1% agarose
gel electrophoresis.
With respect to the effect of immunization and production of
IgY, experimental procedures and similar results were reported
by Gassmann et al. [31], in which chickens were immunized with
20 to 30 mg of mammalian protein added to Freud’s complete
adjuvant. The results of 62 eggs extracted total amount of 4.0
grams of IgY and obtained as a final yield of approximately 130mg
specific for a protein of mammalian cell cycle regulatory antibodies.
Using the immunoblot technique identified that 20 days after
immunization, IgY antibodies appeared, reaching a maximum
production within 30 days after first immunization, maintaining
a good level of production until 81 days, when the average was
72mg of IgY per yolk. These researchers obtained at the end of
the experiment the mean concentration with standard deviation of7.44 ± 0.27 mg of IgY/mL of yolk. For Meenatchisundaram et al.
[32], IgY concentrations ranged from 0.85 to 7.60 mg/mL of egg
yolk, throughout the immunization period. Already to Pauly et al.
[33] variations IgY were between 3.0 to 8.0 mg/ml of yolk. Chui et
al. [34] reached a mean value of 5.75 mg of IgY per ml of egg yolk
immunized as Barbas III et al. [29] had concentrations range from
55 to 80 mg/egg yolk and 75% purity using the same extraction
kit used in this study. In this study, average concentrations of finals
were obtained 3.68 ± .16 mg of IgY/mL in the yolk. One advantage
of the IgY antibody production is that the required amount of
antigen for high and long lasting for IgY yolk of immunized hens
titration would be very low compared to immunization of other
animals such as the rabbit Song et al.[35]; Hatta et al.[36]. When
the result of IgY extract was applied to 6% polyacrylamide gel
was characterized by bands at approximately 212kDa molecular
weight. The literature describes this mass ranging from 180kDa
immunoglobulin Meenatchisundaramet al. [32]; Warret al. [37];
Chalghoumiet al. [38] to 220 kDa Hamada et al. [39].
Andris-Widhopf et al. [40] have also obtained cDNA fragments of approximately 750pb as a result of Overlap PCR reaction scFv fragments. These researchers used chickens immunized with a conjugate of bovine serum albumin (BSA) plus fluorescent and hapten reported as a result of the joining VLand VH, comprising the formation of a linker sequence (VL-linker-VH). They also emphasized that the VL-linker-VHproduct, corresponds to the scFvregion of immunoglobulin, has good applicability for use as befits its use with vector transfection.
In the bursa of Fabricius, the level of the complementarity determining regions (CDRs) of scFv fragments (light and heavy chain) derived from immunoglobulin G (IgG) from chicken and poultry in general, such mechanisms occur: the gene rearrangement and gene conversion somatic hyper-mutation. These mechanisms promote increased variability in this gene fragments due to the incorporation of genes pseudo and direct, acting in the maturation cell of B lymphocytes McCormack & Thompson, [41]; Paramithiotis et al. [42]; Arakawa [43]. Thus the Overlap PCR allows the tool the final product, or the scFv fragment, a great increase in respect of gene variability, since it favors the increase of the combination of variable gene fragments, allowing the number of clones from a library of scFv generated by this method reaches to 1012units Barbas III et al. [29].
cDNA libraries are indispensable to analyze the expression and function of genes and also in the study of protein function, this expression products. Hese information can be of great importance in understanding when and how certain genes are expressed in an cell type or organism. The continuous progress in the development of constitutive techniques of scFvgene libraries and the interest in their applications suggest that these tools will continue to be an important factor for the development of new recombinant biomolecules.
Conclusion
Acknowledgement
For more Lupine
Publishers Open Access Journals Please visit our website https://lupinepublishersgroup.com/
For more Journal
of Veterinary Science articles Please Click Here: https://lupinepublishers.com/dairy-veterinary-science-journal/
To Know more about Open
access Publishers, click on Lupine
Publishers.
No comments:
Post a Comment
Note: only a member of this blog may post a comment.