Lupine Publishers | Journal of Veterinary Science
Forty five Staphylococcus aureus isolated from cases of bovine
mastitis were subjected to Molecular typing by Polymerase chain reaction
to determine their capsular polysaccaharide type. Of the 45 isolates,
33 were confirmed to carry a cap5 locus and cap8 locus was detected in
remaining 12 isolates. To the best of our knowledge this is the first
report of capsular polysaccharide typing of S.aureus isolates from India
Introduction
S. aureus produces a variety of extracellular and cell wall
associated components which are involved in the pathogenesis of bovine,
ovine and caprine mastitis [1].
S. aureus strains produce capsular polysaccharide (CP)
in-vivo
[2] or under defined culture conditions [3]. Although capsule
production of staphylococci was first recognized in 1930 [4], the
prevalence of encapsulation among
S. aureus has been appreciated
only recently. Eleven capsular polysaccharide serotypes have been
proposed on the basis of agglutinating reactivity with adsorbed rabbit
antiserum and precipitation in double immuno diffusion [5,6]. Of these
capsular serotypes 5 and 8 are the most predominant serotypes in human
and animal
S. aureus infections.
Studies on the prevalence of encapsulated strains in bovines shows
the considerable variability that exist in the prevalence of serotype 5
and 8 capsules among bovine mammary isolates of
S. aureus from different countries (Tollerstud et al., 2000). Moreover, the presence of
S. aureus in raw milk is a public health problem, because it was reported that 95% of
S. aureus isolates from bovine mastitis were either CP5 or CP8 in Norway [7]. For effective control of bovine mastitis caused by
S. aureus
in a particular geographical location, a careful characterization of
the prevalent strains in the target population is essential [6]. Studies
on capsular serotyping of isolates are important for the rational
design of mastitis vaccines, containing staphylococcal capsular
antigens. If improved vaccines against bovine mastitis are to be
generated, more studies are required to elucidate the role of these
polysaccharides in the pathogenesis of bovine mastitis [7].
However, capsular serotyping employing conventional techniques fails to identify non encapsulated strains of
S. aureus.
Hence DNA based technique for differentiation of serotypes provide an
alternative to conventional serotyping and has a potential to overcome
the problems associated with the current serotyping techniques which
relay on inconsistent expression of phenotypic traits [7-9]. No data
regarding the prevalence of capsular serotypes of
S. aureus
causing bovine mastitis is available in India. The proposed study would
help in understanding the prevalence of capsular serotypes of
S. aureus in Puducherry, India. This data would help in formulating vaccine based strategies for control of mastitis.
Materials and Methods
Cultures used in the study. Forty five Staphylococcus aureus obtained
from the milk of dairy cattle with clinical and subclinical mastitis in
and around Puducherry, India and
S. aureus strain Reynolds (Capsular polysaccharide type 5) and
S. aureus
strain Wrights (Capsular polysaccharide type 8) were used as standard
reference for identification of Capsular polysaccharide types of
S. aureus by PCR Identification of
S. aureus isolates [10]. The
S. aureus
isolates were initially selected on the basis of colony appearance and a
positive tube coagulase test and their identity was verified by [8] and
Garrity et al. [9]. Their identity was confirmed by PCR using the
primer pairs targeting the nuc gene of
S. aureus. DNA extraction.
Strains were grown on Luria broth 37 °C overnight. Genomic DNA was
extracted with a standard phenol-chloroform procedure as described
elsewhere [10]. Detection of capsular genotype by PCR. The PCR assay for
typing of capsular polysaccharide S.aureus was carried out as described
by Verdier et al [11]. Genomic DNA was used as a template for PCR
amplification with the primers Cap5 k1
(5’-GTCAAAGATTATGTGATGCTACTGAG-3’) and Cap5 k2
(5’-ACTTCGAATATAAACTTGAATCAATGTTATACAG-3’) located in cap5k for capsular
type 5 and the primers Capsule 8 k1 (5'GCCTTATGTTAGGTGATAAACC-3') and
Capsule 8 k2 (5'-GGAAAAACACTATCATAGCAGG-3') located in cap8I for
capsular type 8. The PCR amplification was carried out in an automated
thermal cycler (Eppendorf mastercycler, Germany) according to the
following programme. initial denaturation at 94 OC for 4min followed by
25 cycles of denaturation at 94 OC for 30seconds , annealing at 55 OC
for 30sec and extension at 72 OC for 1min and final extension at 72 OC
for 5min. The amplified products were analysed on agarose gels along
with positive control, negative control and molecular size marker (100bp
la).
Results and Discussion
All the 45 isolates
S. aureus were subjected to molecular
typing using the primer pairs targeting the cap5 locus and cap8 locus of
capsular polysaccharide of
S. aureus. The primer pairs successfully amplified the DNA prepared from the field isolates of
S. aureus
as well as the DNA prepared from the reference cultures used in the
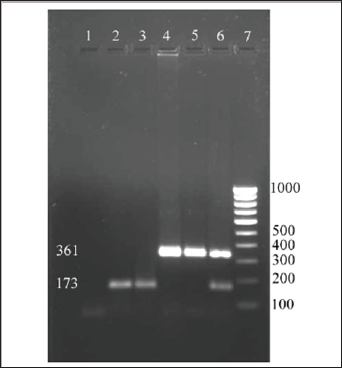
study. The sizes of the amplicons were 361bp for capsular type 5 and
173bp for capsular type 8 (Figure 1). Among the 45
S. aureus
isolates subjected for PCR with CP5 and CP8 primers, 33 isolates were
confirmed to be CP5 strain and 12 isolates were confirmed to be CP8
strain. Out of the 45 isolates, 73.33% were found to carry the cap5
locus and 26.66 % were found to carry the cap8 locus. Poutrel et al. [1]
reported that a majority of
S. aureus from cases of mastitis strains belong to serotype 5 (CP5) and 8 (CP8). In their study they used monoclonal antibodies to
S. aureus
capsular polysaccharide types 5 and 8 to serotype the isolates by
enzyme- linked immunosorbent assay and showed that 69% of 212 isolates
recovered from cow's milk in France were serotype 5 (51%) and serotype 8
(18%) and 30.6% were non-typeable [12].
Figure 1: Screening field isolates of
S.aureus for capsular types.
Tollersud et al. (2000) have showed the variability in prevalence of serotype 5 and 8 capsules among bovine mammary isolates of
S. aureus
from different countries. They performed immunoblot assay using CP5 and
CP8 antibodies and isolates that consistently giving weak reactions
with antibodies to CP5 and CP8 were further evaluated by immunodiffusion
or ELISA. Capsular serotyping of 274 bovine mastitis isolates of
S. aureus
from Europe, showed that the majority of isolates from Denmark (23 out
of 39 isolates), Sweden (29 out of 38 isolates) and Ireland (62 out of
101 isolates), were of serotype 8 [13]. Isolates from Iceland showed an
equal distribution of serotype 5 (10 isolates), serotype 8 (13 isolates)
and non-typeable isolates (11 isolates), whereas in Finland half of the
isolates (32 out of 62 isolates) tested were non-typeable. Serotyping
of the U.S. isolates revealed that only 42% of 362 isolates from seven
different states were typeable with the available antisera and showed 27
% of the isolates were serotype 8 strains and 15 % were serotype 5
strains, but the majority (58%) of U.S. isolates were non-typeable.
Strains of
S. aureus that do not react with antibodies to CP5
or CP8 are referred to as non-typeable (NT) by conventional serotyping.
Karakawa et al. [12] and Lee et al. [13] reported that these NT strains
also fail to react with specific antibodies to serotype 1 or 2 CP. This
is one of the problems encountered in the conventional serotyping of
S.aureus. Han et al. [13] reported the usefulness of monoclonal
antibodies reactive with the type 5 and 8 CP in characterizing
S. aureus
from clinical isolates that monoclonal antibodies have been described,
and has also been demonstrated. Monoclonal antibodies for CP5, CP 8 and
336 were used to characterize 107 isolates of
S. aureus [14-15].
Forty six per cent of them were typed as 336, while serotype 5 and 8
accounted for 12.1% each. The rest were declared as non-typeable.
However O'Brien et al. [14] and Ma et al. [15] reported that Type 336
isolates do not express capsule but do express cell surface
polysaccharide or the 336 polysaccharide (336PS), which resembles
S. aureus
cell wall teichoic acid and hence not a true capsular type. In order to
avoid the problems encountered in the conventional serotyping, a PCR
method was developed by Verdier et al. [11] to detect capsular types of
S. aureus.
In their study using the rabbit polyclonal antibodies specific to
capsular polysaccharide types 5 and 8, 81 of the 195 isolates were
capsular serotype 5 (T5) (42%), 88 were capsular serotype 8 (T8) (45%),
and 26 (13%) were non-typeable. A PCR method was developed to detect
capsular type of
S. aureus isolates since serotyping method
allowed typing of only 87% of strains (169 of 195). All strains included
in the study have been investigated by PCR. But PCR method allowed
genotyping of 100% strains [16-18].
Their study revealed that all
S. aureus clinical isolates
included in the study carried either the cap5 (46% of cases) or the cap8
(54% of cases) locus by PCR method, and demonstrated that the capsular
phenotype that was determined by conventional serotyping method was
confirmed by PCR. However, all 336 serotype strains that reacted
specifically with 336 antibodies but not with capsular polysaccharide
type 5 or 8 antibodies, carried the cap8 or cap5 genes (cap8 18 and 8
cap5 isolates). This study revealed the predominate capsular
polysaccharide types prevailing among the bovine S.aureus isolates was
the CP5 compared to CP8. Data on the
S. aureus capsular
polysaccharide types will help in formulating vaccine based strategies
for the effective control of bovine mastitis due to
S. aureus.

No comments:
Post a Comment
Note: only a member of this blog may post a comment.