Lupine Publishers | Journal of Textile and Fashion Designing
Abstract
Introduction
Several workers have reported blending of pineapple leaf fibres alone or in blends with other fibres [1-4], but scanty work has been reported on the analysis of PALF blends since the early work by Dey et al [5]. Numerous methods are available for analyzing fibre blend composition, of which the chemical method is commonly employed when a sufficient sample is available. It is more desirable to opt for a micro technique that requires smaller samples of the order of a few milligrams. Such samples can be analyzed unambiguously in order to identify variations in blend composition from place to place for quality control and investigation of fabric defects that cause streaks or depth-of-shade variations in fabric.
Polyester and cotton or wool and acrylic fibres are complimentary to each other due to their versatility. Since PALF is often blended with acrylic fibres to improve performance and hand, information on specific changes that occur during blending should be of interest. The infrared spectrophotometer is a vital instrument for both qualitative and Quantative analysis of polymer blends. The occurrence of non-overlapping absorption bands in IR spectra makes such analysis easier and more reliable. Various examples of quantitative determinations of fibre blends have been reported including ramie/acrylic by Dey et al, cotton/Dacron by O’Conner [6], wool/ Terylene by Clark and Hickie [7], cotton/polyester by BhamaIyer et al. [8], and acrylic/wool by Soosamma et al [9]. In this paper, the authors report a simple technique based on an IR method for quantitative analysis of PALF/acrylic blends.
Materials and Methods
Sample preparation and calibration plot
Acrylic and PALF fibres were finely cut with scissors and sieved through a 100 mesh screen. Acrylic/pineapple leaf fibre blends of predetermined compositions were prepared by carefully weighing finely cut fibres. The fibre mixture was mixed thoroughly with 400 mg spectral grade KBr powder by grinding for 5 minutes with a mortar and pestle. The sample-KBr mixture was placed in a die and pressed for 5 minutes under a pressure of 421.85 Kg/cm2. The sample was also subjected to evacuation in the die for 5 minutes prior to pressing. The pellets were taken from the die and mounted on a magnetic pellet holder. These pellets transmitted in the range of 20-80% while being scanned. A standard calibration plot was obtained by measuring the absorbance of the nitrile (C≡N) band.a) Blend analysis: The same procedure was followed to analyze the blend composition of five different blended yarn samples. A known quantity of the sample was powdered thoroughly and mixed well and 4 mg of the sample was weighed accurately and mixed with 4oo mg of KBr. For each sample, two sets of pellets were prepared as described earlier (Figure 1).
Figure 1: Calibration Plot for determination of Acrylic
content in Pineapple leaf fibre/acrylic blends by
measurement of Absorbance of (C≡N) band.

b) Spectra Recording: Spectra of the Pellets were
recorded by a Shimadzu double beam model IR PRESTIGE-21
spectrophotometer from 4000 to 400 cm-1 under a normal slit
program and a scanning speed of nearly 19s/100cm-1. A KBr pellet
without the sample was used in the reference beam. For each pellet,
the nitrile band at 2243 cm-1 was scanned twice, the second scan
corresponding to rotation of the pellet through 90°in its own plane
with reference to the first position. If the transmittance differed by
more than 1%, this was taken to indicate no uniformity of mixing
the samples. Fresh pellets were prepared again discarding such
samples and their spectrum was recorded. The peak intensity
(absorbance) in each case was measured by the baseline technique
[11].
c) Chemical Analysis of Blends: The actual blend proportions were determined chemically as per the method IS: 3421-1966 for blend estimation [12]. All samples were extracted with a benzene-methanol (3:2) mixture to remove oil and any finishing materials that had been added during processing. Each blended sample was analyzed twice and the average value was reported in Table 1. Dey et al reported that the blend compositions of ramie-acrylic blends can be unambiguously assessed using infrared spectra with the help of calibration plot15. These compositions match those obtained by chemical methods (Table 1).
Results and Discussion
Table 1: Blend Extimation by Chemical Method.
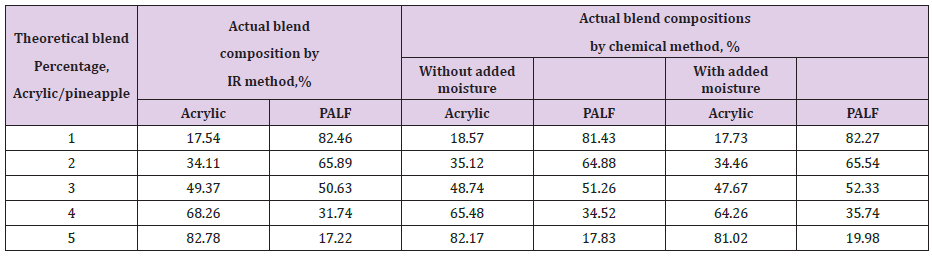

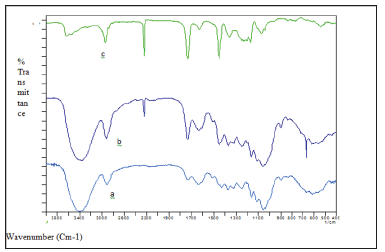
Figure 2: Infrared spectra of (a) PALF, (b) 50/50 PALF/
acrylic, and (c) acrylic fibre.
The absorbance of the C=O band versus composition does not
yield a straight line. Soosamma et al. reported in their earlier work
that the intensity of the (C≡N) band remains unchanged during
ball milling period. This indicates that below a certain particle size,
ball milling does not have any effect on the (C≡N) band. Hence,
the absorption of the (C≡N) band versus composition gives a
straight line. From such a calibration plot, the actual composition
of any blend sample can be obtained rapidly with high accuracy.
Blend compositions of the yarns based on the theoretical blend
compositions of PALF/acrylic yarns spun on the jute spinning
system measured by means of calibration plots and chemical
methods are enumerated in Table 1. Figure 2 reveals the nature
of the infrared spectra of PALF, 50%/50% PALF/acrylic blend and
acrylic fibres. The results obtained with the infrared method using
a calibration graph show the true compositions as given by the
chemical method to a high degree of accuracy. After gaining some
familiarity with the spectra of fibres, IR spectroscopy can provide a
good technique for estimating blend composition-rapidly, reliably
and with reasonable sensitivity.
Conclusion
Acknowledgement
For more Lupine Publishers Open Access Journals Please visit our
website:
For more fashion and textile research journal articles Please Click Here:
To Know More About Open Access Publishers Please Click on Lupine Publishers
Follow on Linkedin : https://www.linkedin.com/company/lupinepublishers
Follow on Twitter : https://twitter.com/lupine_online
No comments:
Post a Comment
Note: only a member of this blog may post a comment.