Lupine Publishers | Journal of Immunology & Infectious Diseases
Opinion
Innovation in technology is the key element to modernize medical treatment and will have a significant impact on all phrases of our lives, particularly the healthcare upon aging population. Many diseases have the common root on immunology, particularly inflammation, and the current advanced immunotherapy has all based on the biological approach like cytokines to alter the immune response for achieving better therapeutic outcomes. At here, I would like to propose a new multidisciplinary strategy as a new alternative for treating immuno-based diseases via the use of a novel family of tunable immune response (TIR) biomaterial platform technology. The theoretical base of this new alternative strategy is to modulate the immune cell (i.e., macrophage) associated inflammation via the feeding of different types of L-arginine (Arg)-based biodegradable polymers to macrophage to control the direction of the dual Arg metabolic pathways by macrophage. It is well-known that macrophage can uptake Arg and metabolize it either through the iNOS or Arginase pathways. The iNOS pathway would lead to the production of NO, and hence cytotoxic (M1 pro-inflammatory phenotype), while the arginase pathway would lead to the formation of polyamines and L-proline, and hence tissue regeneration/repair (M2 anti-inflammatory phenotype). It is my opinion that the use of the macrophage’s Arg metabolic pathways to tune immuno-responsive character of macrophage is a better strategy than the conventional use of cytokines for either classical or alternative macrophage activation. This is because
I.Cytokines have a much shorter life span and can be easily deactivated in real clinical applications, while the Arg-based Tunable Immune Responsive (TIR) biomaterial platform technology strategy can have a very long lifespan and shelf life, i.e., lower healthcare cost and more sustained efficacy.
II.The Arg-based TIR biomaterials can be formulated into a variety of physical forms like nanoparticles, fibrous membranes, melt-spun fibers, 3D microporous hydrogels, films, coating etc., while the cytokines can’t be formulated into any form, except their native form. The capability of having a variety of physical forms can definitely facilitate and expand their clinical applications. For example, TIR-based nanoparticles can couple with drugs to provide a synergistic therapeutic effect toward disease treatment. Such an integration of macrophage-proactive TIR-biomaterials with drugs will open a new dimension of immunotherapy.
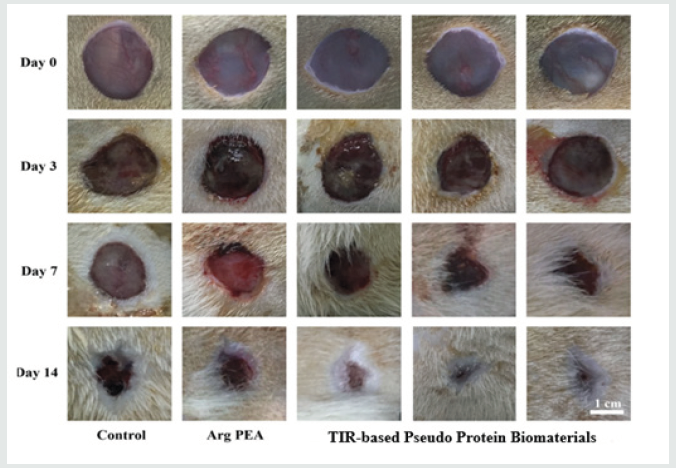
This new family of TIR-capable biodegradable biomaterials have a nickname, “TIR-capable Pseudo-protein biomaterials” because they are built from 3 building blocks, amino acids, alcohols and acids, and possess both proteins and non-protein properties. Because of the enormous variety of these 3 building blocks, these pseudo-protein biomaterials can be tailored designed for meeting specific clinical needs, hence have become a platform biomaterial technology. The 1st generation of these new pseudo-protein biomaterials has been licensed and approved as the coating material for a new drug-eluting stent (Slender IDS®) for human commercial use in Europe in 2016 to treat the blockage of coronary artery. Very recently, Chu has teamed up with the Institute of Chinese Medicine in the Hong Kong Baptist University to pursue the use of the newly developed pseudo-protein nanotechnology as the delivery vehicles for Chinese medicine like gambogic acid to treat the most challenging and difficult triple negative breast cancer (TNBC). Their animal data are very encouraging and promising. This infusion of a new western pseudo-protein nanotechnology from Cornell with ancient Chinese medicine is believed to be able to modernize Chinese medicine-based treatment significantly for a far better therapeutic efficacy and lower toxicity. My lab has recently also collaborated with the National Engineering Research Center for Tissue Restoration and Reconstruction and School of Materials Science and Engineering in the South China University of Technology, Guangzhou to test the feasibility of this new TIR-based pseudo-protein biomaterials for treating diabetic burn wounds in diabetic-induced mice as diabetic patients are well-known to have the inherent difficulty to heal their wounds timely. Burn wounds are well-known for their excessive inflammatory character. Therefore, a combined diabetic burn wound appears to provide the most challenge animal model to test the potential of my lab newly developed strategy of TIR-based pseudo protein biomaterial technology for immunotherapy of a variety of diseases. The animal trial data show that this novel TIRbased pseudo-protein biomaterials can indeed timely promote the healing of the 3rd degree burn wounds in diabetic-induced mice, and the mechanism behind this very promising animal data is the TIR-based pseudo-protein biomaterials induced transformation of the highly inflammatory M1 macrophage phenotype in the diabetic burn wound bed into anti-inflammatory M2 macrophage phenotype. Figure 1 shows the wound healing performance of a diabetic 3rd degree burn wound mice after the topical treatment by my lab TIR-based pseudo-protein biomaterials.
Figure 1:
Read
More About Lupine Publishers Journal
of Immunology Please Click on Below Link:
https://lupine-immunology-infectious-disease.blogspot.com/

No comments:
Post a Comment
Note: only a member of this blog may post a comment.