Lupine Publishers| Journal of Nanomedicine
Abstract
The present study was aimed to account a green synthesis of copper nanoparticle is by interaction of leaf extract and copper salt. The bio-synthesis of nanoparticles put forward a cost free and eco-friendly method of nanoparticle synthesis. Copper nanoparticles were synthesized by using aqueous solution of copper sulphate and extract of Aloe barbadensis. The prepared leaf extract was observed when 1mM copper sulphate solution is added in it. Color change of the reaction mixture was observed from deep blue to colorless and then to brick red and dark red indicating the formation of copper nanoparticles. The synthesized CuO NPs was characterized by using different technique such as UV, IR, XRD, and SEM. ward a cost-free and environmentally suitable method of nanoparticle synthesis. Synthesized CuO nanoparticles with average particle size of 60n. Shape of copper nanoparticles was spherical and cubic and their range was 80-120nm Different functional group in synthesized nanoparticles are examined by FTIR. UV spectrophotometer confirm peak of copper nanoparticle experiments Copper oxide nanoparticles shows maximum absorbance at 272nm. Catalytic activity of synthesized nano particles is also examined on the degradation of malachite green. This catalytic effect of copper oxide nanoparticles can be contributed to its small size.
Keywords: Aloe Barbadensis, SEM, Copper Oxide Nanoparticles, Green Synthesis, XRD, Green Synthesis, Highly Stabilized Nanoparticles , Ecofriendly, Phenolic Content, Degradation of Malachite Green
Introduction
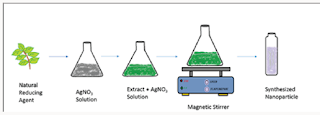
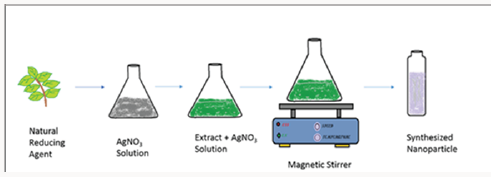
Nanotechnology deals with manipulation of matter at low size normally lesser then that of the 100nm. Metallic nanoparticles can be prepared by chemical and physical method. These methods have certain flaws like toxic chemicals and also dangerous to environment [1]. Developing research in green chemistry employed prominent part in nanotechnology to attain benefit to society surface area and mass ratios increase adsorption property [2]. Green synthesis has been concerned in synthesis of highly stabilized nanoparticles. Synthesis of nanoparticles taking assistance of ecofriendly methods has achieved huge attention in the modern era. The particles produced by green synthesis differ from those using physio–chemical approaches. [3] Green synthesis, a bottom up approach, is similar to chemical reduction where an expensive chemical reducing agent is replaced by extract of a natural product such as leaves of trees/crops or fruits for the synthesis of metal or metal oxide NPs. Biological entities possess a huge potential for the production of NPs. Biogenic reduction of metal precursors to corresponding NPs is eco-friendly [4] (Figure 1). Copper nanoparticles were synthesized by leaf extract of Aloe barbadensis plant. The plant is also known as “Aloe vera”. The green synthesis of copper nanoparticle by Aloe vera plant extract is fast, easy and (Figure 2). environmentally suitable method [5].
Phenolic content in plant extract dissolved in water, degradable and catalyzed synthesis of nanoparticle as capping and reducing agent [6]. This old plant is well known for deeper healing effects. It is majorly present in cosmetics and skin creams. It has the ability to clean skin and anti-aging effects of it is also famous [7]. Aloe vera contains antioxidant vitamins A, C, and plus vitamin B12, folic acid, and choline [8]. Its gel juice is taken as power drink. Minerals such as calcium, copper, selenium, chromium, manganese, magnesium, potassium and zinc are present in aloe vera. Leaves of aloe vera provide anthraquinones [9]. Copper nanoparticles synthesis by using electron microscopy represented that their range is upto 50 to 130nm [10]. Copper nanoparticles important as compares to other nanoparticles due to their properties that are found at less cost than that of expensive metal such as gold and silver such as examination of catalytic activity of copper nanoparticles by degradation of malachite green [11]. Copper oxide particles show effective catalytic removal of organic dyes such as malachite green, when particles were added into it [12]. The use 0fgreen method increased so much because of its easy preparation and low manufacturing cost. Moreover, less to toxic starting materials and ease of handling make it more favorable [13]. Malachite green is extensively used in many industries as a dye for leather, textiles and also in aquaculture industry to control fish parasites and disease [14]. Malachite green is classified as a class II health hazard and they pose toxicity (mutagenicity, genotoxicity) to the aquatic organisms like fish, algae, bacteria etc. and it’s proved to be highly carcinogenic and is banned by many countries [15] (Figure 3). The removal of organic pollutants and dyes from industries remain as a challenge as these dye molecules are difficult to decompose. Varieties of organic and heavy metal pollutants were removed by nano adsorbents by various research groups [16].
Materials and Methods
Material
Copper sulphate, Aloe barbadensis leaves, sodium borohydride (NaBH4), organic dyes such as Malachite green
Preparation of Plant Leaf Extract
50g of the Aloe vera is taken from the nearby garden. The leaves of aloe vera are first separated from the gel part o0f aloe vera. The leaves are then washed thoroughly with distilled water to remove soil and dust particles. After washing leaves were dried and finely chopped. These finely chopped leaves were allowed to boil for 15min at 100 °C with 100mL of de-ionized water in a250-mL flask and then allow to cooled down to come at least at room temperature. The resulting solution is passed through a filter paper to remove any solid particles and then again filtered throughaWhatmanfilterpaperofporesize0.2μm.Thefiltrate is stored at 6 °C as a stock for the synthesis of CuO NPs.
Green Synthesis of Cuo NPs: A copper sulphate solution of fifty milliliters was added to 15ml aloe vera leaves extract. The solution was stirred on a magnetic stirrer at 120degrees. The color change was observed. The color changes from deeply blue to colorless and then dark red at saturation. Brick red color confirms the nanoparticles formation. The resultant solution was centrifuged for ten mints at speed of 50,000rpm. After discarding supernatant copper oxide nanoparticles were dried in a watch glass. After drying, black color particles (Figure 4). were assemble for further characterization.
Characterization of Green Synthesis Copper Nanoparticles
The morphological, structural and chemical composition of CuO NPs were analysed by using SEM (jsm-6480) and XRD (XPERTPRO) equipment. Optical properties of synthesized particles are investigated by UV spectrophotometer (DB-20). Size and shape of copper oxide nanoparticles were observed by SEM (jsm-6480). The crystal structure of synthesized nanoparticle is examined by XRD (XPERT-PRO), FTIR analysis is performed for the collection of the functional groups, present in this synthesis of CuONps.
Colour change observation: Color changes indicate the formation of nanoparticles of copper oxide. Blue color solution was turned into red or brick red indicated for formation of copper nanoparticles synthesis.
Result
X-Rays Diffraction Studies
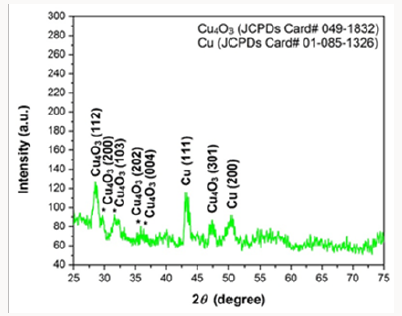
Copper oxide nanoparticles were examined by X-ray diffractometer (XPERT-PRO). Copper oxide powder was put in cubes of XRD for calculation of intensity. The resultant pattern of synthesized nanoparticles was analyzed. The peaks at 2θ correspond to intensity as the peaks at28, 29.8, 32.1, 35.8, 36, 43.3, 47.5, 51.1, and have 112, 200, 103, 202, 004, 111, 301 and 200, pattern which is compare to JCPDS card no (049-1832). The pattern of Cu nanoparticles compared to JCPDS card no (01-085-1326), the peaks at 2θ. XRD pattern confirmed that CuO nanoparticles are highly crystalline with cubic crystal structure. The average size of the particle calculated by Scherrer equation was 60-100nm (Figure 5).
FTIR Analysis
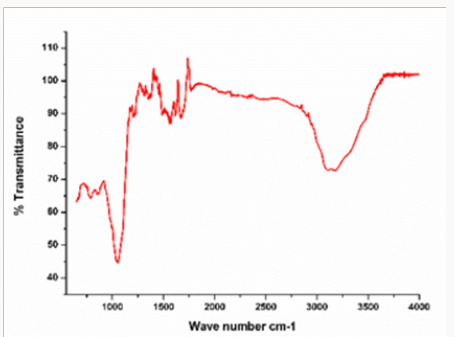
In this analysis, FTIR (IPRrestige-21) spectrum was analyzed. The analyzation confirms the presence of copper nanoparticles. Different peaks were observed at 1100cm-1 confirm formation of Copper oxide nano particle speaks was observe in range of 400-4000cm-1. The FTIR spectrum of Copper oxide nanoparticle exhibits that the broad absorption band at 32cm-1 corresponds to the hydroxyl (OH) functional group in alcohols and phenolic compounds. The peak at 1601.2cm-1 is due C=C aromatic bendindg. Absorption peak at 1038.0 cm-1 stretching vibration of C–O group of primary and secondary alcohols (C–O), while smaller peaks at 900- 700cm-1 were also (Figure 6). Assigned to the aromatic bending vibration of C–H group (Table 1).
Table 1: Absorption peak at 1038.0cm−1 stretching vibration of C–O group of primary and secondary alcohols (C–O), while smaller peaks at 900–700 cm−1 were also assigned to the aromatic bending vibration of C–H group.

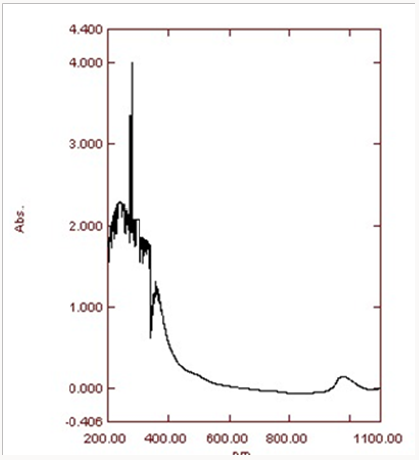
Ultra violet spectroscopy: The presence of copper oxide nanoparticles is confirmed at the range of 200-1100nm. The ecofriendly method for the synthesis of copper oxide nanoparticles using Aloe vera leaves extract proved feasible, coast free and successful method. UV-Vis spectra analysis has apparently shown the formation of copper oxide nanoparticles. Nanoparticles synthesized have variety of application in the different field. The maximum absorption peak is between 265-285nm.The peak at about 280nm was achieved (Figure 7).This peak confirmed formation of the copper oxide nanoparticles.
SEM Analysis
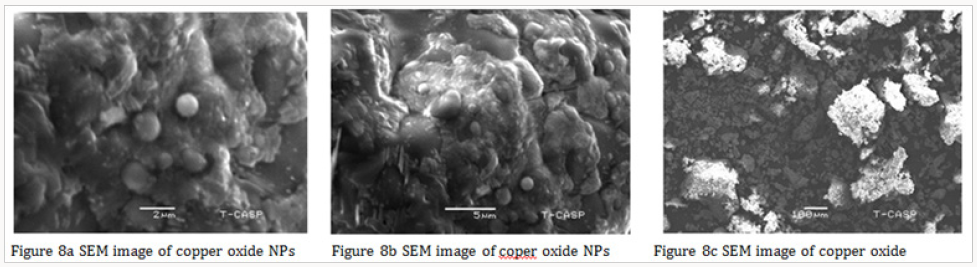
The average particle size of copper nanoparticle was analyzed by SEM model (JSM-6480). The range of grain of copper oxide nanoparticle was calculated about 50.5-130nm by SEM micrograph. It was observed that particles were smooth with a spherical shape (Figure 8). The catalytic activity of the CuO NPs analyzed by the degradation of malachite green dye. The catalytic activity of the CuO NPs analyzed by the degradation of malachite green dye. Preparation of 1000mg/l dye S.S. 1000ppm solution of Malachite green dye was prepared by dissolving dye in 1-liter distilled water.
Different concentration of dyes was prepared from stock solution. 100ppm solution was prepared from 1000ppm solution after dilution. After that 150,200,250-ppm solution was prepared. 18g of NaBH4 is made up to 10mL and kept aside. Different concentrations of NaBH4 and catalyst are tested on the methylene blue dye. The catalytic degradation of organic dyes was observed by measuring UV-Visible spectra at regular time intervals.
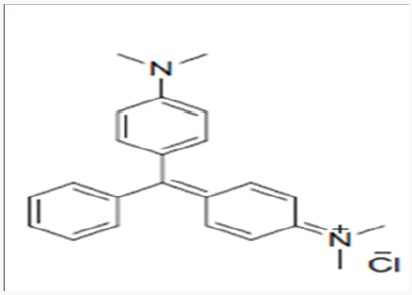
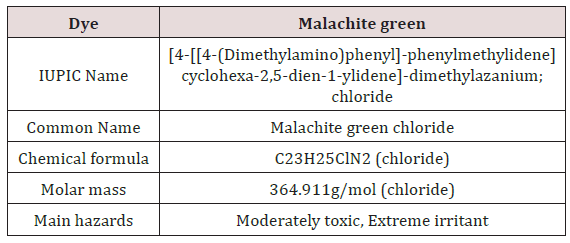
Malachite Green: Malachite green is extensively used in many industries as a dye for leather, textiles and also in aquaculture industry to control fish parasites and disease. The use has increased so much because of its easy preparation and low manufacturing cost (Table 2) (Figure 9).
Structure
Dye Degradation: The degradation of malachite green in the absence and presence of CuO NPs were studied spectrotometrically by using DB-20 UV-Vis spectrophotometer determining the decrease in the absorbance at 631nm.The reaction was study spectrophotometrically at room temperature (25 0C). The colour of the reaction mixtures faded, indicating that degradation had occurred. The same procedure was followed for uncatalyzed reactions, in absence of CuO NPs.
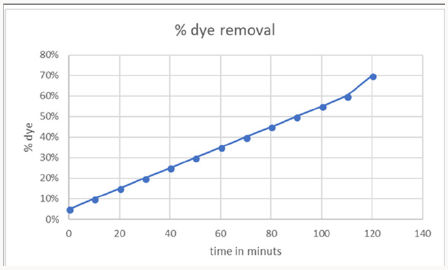
1-Time Effect on Dye Removal: Decolorization of dye Malachite Green at room temperature was analyzed. Initially 20ml dye solution was taken and 1mg of greenly synthesized copper nanoparticles using aloe vera leaves extract dissolved in it. 0.1mg of NaBH4 was dissolved as a reducing agent. The solution was heated for 10- 20 mint at 100 degrees. The time interval was taken in consider gradually during reaction. The removal percentage of decolorization was calculated and draws graphically. The maximum time was 120 mints with70% color removal. This confirms the rapid reaction of copper oxide nanoparticles (CuO NPS) (Figure 10).
Figure 11: Aloe vera synthesized copper oxide nanoparticles showed maximum percentage decolorization as pH was increased at a certain limit after more increase has a negative effect.

2-pH effect on dye removal: pH of the solution also majorly affected the de-colorization of dye. pH effect on the decolorization of copper oxide nanoparticles was analyzed in this research. Aloe vera synthesized copper oxide nanoparticles showed maximum percentage de-colorization as pH was increased at a certain limit after more increase has a negative effect. This may be happened due to the formation of more positive ion competition. Maximum de-colorization 70% was at pH 5 (Figure 11).
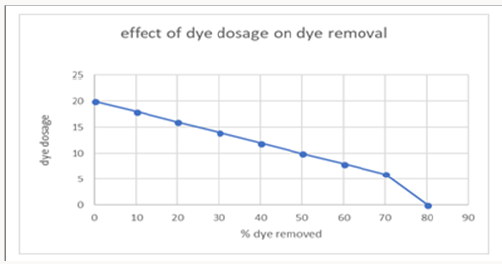
3-Concentration of dye effect on decolorization of dye: The increase or decrease in the concentration of Malachite green MG dye is also considerable in decolorization efficiency. The graph was obtained after experimenting various concentration of dyes. The maximum amount of dye taken was 20mg/l. After increasing concentration no effect on 70 decolorizatioof dye was observed (Figure 12).
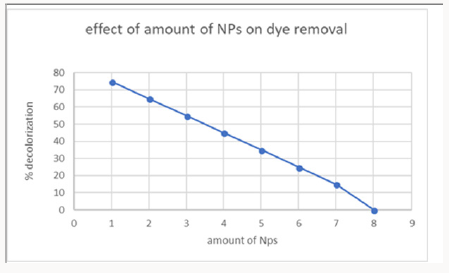
4-Effect of Copper Oxide Nanoparticles Amount on Dye Removal: The number of copper oxide nanoparticles exhibits positive results on decolorization. The number of nanoparticles 1 gram was taken showed maximum de-colorization power. This confirmed from the experiments that increasing of nanoparticle showed no effect on de-colorization. This concentration of nanoparticles was used in further experimentation of research (Figure 13).
Conclusion
Here in conclusion, we concluded a method of green synthesis of Cu nanoparticles by leaf extract of Aloverabarbadensis plant. This eco-friendly way of synthesis of nanoparticles is more recommended over other methods as green synthesized CuO NPs are cost-effective, biogenic molecules with the capability to serve as dye absorbent. From vast of analyzation on nanotechnology for synthesis of nanoparticles it is declared that it is safer and best by using natural plants. With the huge plant variety much more plants are still not known for the synthesis of nanoparticles. Nanoparticles synthesized can be applicable in the different field of biochemistry, Pharma, agriculture and industry. Copper oxide nanoparticles have the ability to remove carcinogenic dyes. In the present study, Malachite green dye was removed by nanoparticles and its time, pH, contact time was observed. The maximum contact time was 120min, pH was observed 5, nanoparticle amount 1mg which proved green synthesized copper nanoparticles, as best removal of carcinogenic dye like Malachite green.
Read More About Lupine Publishers Journal of Nanomedicine Please Click on Below Link https://lupine-publishers-nano-science.blogspot.com



No comments:
Post a Comment
Note: only a member of this blog may post a comment.