Lupine Publishers| Journal of Drug Designing & Intellectual Properties
Abstract
The semi interpenetrating network hydrogels (Z1-Z15) were prepared from different ratio of sodium alginate and linear poly (acrylamide-co-diallyl dimethyl ammonium chloride) then they mixed with the acrylamide and bisacrylamide as cross linking agent and polymerized via redox polymerization to form the semi-IPNs. All prepared semi-IPNs were loaded with three different amounts from Gabapentin as drug model. The swelling characteristics were studied for all semi- IPN hydrogels by determining the swelling ratio (Q).The release of Gabapentin was followed by using U.V. spectroscopy at (202 , 201 and 204) nm at constant temperature (37°C) in distilled water , simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) respectly. The results of Gabapentin released indicated that all semi-IPNs have the ability to release drug to environment and the amount of Gabapentin released about 50% during two hours. Higuchi equation was used to determine the Higuchi constant which is very useful to forecasting the amount of Gabapentin released theoretically.
Keywords: Polyelectrolyte; Polymer Drug Delivery; Gabapentin
Abbrevations: SGF: Simulated Gastric Fluid; SIF: Simulated Intestinal Fluid; TDDSs: Targets Drug Delivery Systems; CDDSs: Controlled Drug Delivery Systems; SGF: Systems In Simulated Stomach Fluid; SIF: The Simulated Intestinal Fluid; WU: Water Uptake
Introduction
It is rare to use drugs as pure chemicals alone, it is given as the formula of drug structures such as drug delivery systems. Simple solutions can be developed to form these systems through the use of appropriate additives or excipients to prepare the pharmacological structures. The effectiveness of many of drugs is often designed to working at the site of the therapeutic act, So the method of delivery of medication can have a significant impact on its effectiveness. In many cases conventional medications reach the target location in a small amount while the most amount of the medication is distributed in the whole ofthe body according to its physicochemical and biochemical properties, so it has developed drug delivery systems that improve the drug's pharmacologic action, reduce side effects and toxicity within the body of the organism [1,2]. The transition from simple tablets to tablets of continuous release and the discovery of sophisticated programmable delivery systems led to the delivery of drugs to the target and cells are more accurate [3].
The synergy between chemists, biologists, doctors and biomedical engineers over the past 20 years has led to the development of controlled release technology and has been the best solution for drug delivery systems. It is a type of drug products that is controlled for long periods of time with maintaining drug concentration in the blood Within optimal treatment limits [4,5]. It is justified to resort to these systems instead of traditional medication formulas due to the problems in metabolism or absorption of medication when using traditional systems or to improve the treatment itself and the release of the drug must be in the right part of the body and the rates required for treatment [6,7]. Controlled drug delivery systems are designed to deliver drugs at controlled rates for specified periods of time and release them at required rates in places where treatment is needed. The concept of drug delivery systems can be classified into two categories:
i. Targeting is one of the controlled release systems and works on delivery of the active ingredient only to the required tissue or organs called Targets Drug Delivery Systems (TDDSs). Examples of such systems include the delivery of chemotherapeutic drugs to tumour sites directly without damage to other healthy tissues [8].
ii. The systems that control the rate and speed of release of the active substance are known as Controlled Drug Delivery Systems (CDDSs) [9].
Polymers are the most materials which widely used in controlled release systems because their manufacturing processes are easy and widely used to design drug release systems as well as easy control of their physical and chemical properties during preparation [10,11]. There are several types of controlled release formulations [12], the first type is long-range release system(sustained release), It provides effective concentrations for long periods of time and reduces the repetition of taking doses to improve therapeutic compliance and reduce the need for repeated visits to clinics [13,14]. The second is Prolonged release system; this system reduces the release of the active ingredient, resulting in a reduction in the toxic effects of this substance and maintenance of the therapeutic activity required for it [15,16].The third is Delayed release; This system delays the release and then release of the active substance without hindrance, for example oral capsules that remain in the stomach intact and break down only at the highest acidic function in the small intestine or colon [17]. The fourth is Repeat action dosage forms; this system is designed to release one dose of medication and for a certain period of time the other dose is released and thus [16,18].
This work consist of preparation of a number of new electrolytic gels polymeric compositions adopted in the composition of the presence of (Acryl Amide, Sodium alginate, linear copolymer (Acryl Amide -co-diallyl dimethyl lammonium chloride) as semi interpenetrating polymer network (semi-IPN) with different weight ratio and loading these semi-IPNs with Gabapentin as a pharmacological drug because of its important pharmacological effects to treat cases of epilepsy and neuropathy and finally study the Gabapentin released from the polymer systems in simulated stomach fluid (SGF), the simulated intestinal fluid (SIF)and distilled water.
Experiments
Materials
Chemicals used in this study were supplied from different sources; Gabapentin was supplied from Zhejiang chiral Medicine chemicals Co. Ltd., China, Poly (acrylamide-co-diallyl dimethyl ammonium chloride) solution 10%wt/v), Sodium alginate, N,N\- Methylene bisacrylamide were supplied from Sigma Aldrich, N,N,N\,N\-Tetramethylene ethylene diamine was supplied from Fluka , Acryl amide was supplied from Merck Co. .
Preparation of Semi - IPN Gels (Z15-Z1)
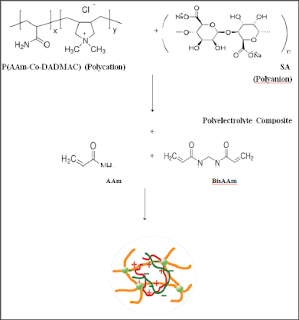
The semi-IPNs (Z1-Z15) which loaded with Gabapentin were prepared from two components. The first constituent consisted of sodium alginate with copolymer (acrylamide-co-diallyl dimethyl ammonium chloride) (AAm-Co-DADMAC) to form polyelectrolyte structure While the second component is cross linked poly acryl amide. 1g of sodium alginate was dissolved in 15 mL of distilled water at 45oC with stirring then 0.2g of Gabapentin,1 mL of copoly (AAm-Co-DADMAC) solution in 10 ml of distilled water were added with stirring until the solution becomes homogenous then2 g of acryl Amide and (0.2 g) of bisacrylamide as cross linker in (5 ml) distilled water were added with stirring and finally 1% sodium per sulphate ( 10% w/v in distilled water) was added as initiator for polymerization reaction and mixed followed by addition 50|il from N,N,N,N-tetra methyl ethylene diamine as accelerator to the decomposition of initiator with stirring for ten minutes to complete the polymerization reaction. The resulting gel is cut into pieces close to the weight and washed with water once and then left to dry at 30 °C and kept in incubator at 20 oC till used. Table 1 shows the quantities that used in the preparation semi-IPN composite hydrogels Z1 to Z15, Scheme 1 shows the chemicals equations for polymerization reactions and Figure 1 showed the photograph of Z1, (Scheme 1).
Read More About Lupine Publishers Journal of Drug Designing & Intellectual Properties Please Click on Below Link: https://lupine-publishers-drug-designing.blogspot.com/
No comments:
Post a Comment
Note: only a member of this blog may post a comment.