Lupine Publishers| Journal of Gastroenterology and Hepatology
Abstract
Background: Non-alcoholic fatty liver disease is not simply the hepatic manifestation of obesity and diabetes but also linked to hepatocellular carcinoma. Yet, its effective treatment has not been established. In this study, we evaluated the effect of a short-term administration of luseogliflozin to patients with type 2 diabetes having non-alcohol fatty liver disease. Luseogliflozin is a unique sodium-glucose cotransporter 2 inhibitor which is metabolized in and excreted by the liver in addition to the kidney. Therefore, the drug might possess an additional effect on other agents of the same class which are exclusively metabolized in the kidney.
Methods: Using alanine aminotransferase >20 IU/L as a diagnostic basis for non-alcoholic fatty liver disease, 19 patients, not taking alcohol, with type 2 diabetes (male/female 15/4, the median age 57 years) was treated with 2.5 mg luseogliflozin for 12 weeks.
Results: Pre- and post-treatment median values for alanine aminotransferase were 51 IU/L and 33 IU/L (p = 0.001), and the corresponding values for the fibrosis index based on the four factors [age (years) ∙ alanine aminotransferase (IU/L)] / [platelets (109/L) ∙ alanine aminotransferase (IU/L)1/2)] were 1.669 and 1.314 (p = 0.043). There was no adverse effect of the drug. Our findings were essentially compatible with the results of the previous studies reviewed.
Conclusion: We conclude that sodium-glucose cotransporter 2 inhibitor could be the choice for the pharmacological treatment of non-alcoholic fatty liver disease. Especially, a short-term administration of it is consistently effective in mild cases.
Keywords: SGLT2 inhibitor; NAFLD; Fibrosis-4 index; GPR-index; APRI.
Abbreviations: SGLT2i: sodium-glucose cotransporter 2 inhibitor; NAFLD: non-alcoholic fatty liver disease; NASH: non-alcoholic steatohepatitis; T2DM: type 2 diabetes mellitus; AST: aspartate aminotransferase; ALT: alanine aminotransferase; GGT: gammaglutamyl transpeptidase; Fib-4 index: Fibrosis-4 index; GPR-index: gamma-glutamyl transpeptidase to platelet ratio; APRI: aspartate aminotransferase to platelet ratio
Introduction
Obesity or overweight is the motherland of non-alcoholic fatty
liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH)
[1]. Accordingly, with increasing trend of body weight worldwide,
the number of patients with the liver problem is relentlessly
increasing [2]. Recently, NASH has also been attracting the attention
as a cause of hepatoma [3,4]. Under such yet, treatment of NAFLD
(NASH and NAFLD are collectively called NAFLD hereafter in this
communication), irrespective of presence or absence of diabetes,
has not been established. Results of treatment of patients with
type 2 diabetes (T2DM) having NAFLD with sodium-glucose cotransporter-
2 inhibitor (SGLT2i) appears promising [3-7], the
effectiveness of a short-term treatment, such as 12 week-treatment,
has not been established.
Here, we evaluated effectiveness of a short-term administration
of SGLT2i, luseogliflozin, that is metabolized not only in the kidney
but in the liver [8]. A mini literature review on this issue was also
performed to resolve the current inconsistency. This study was
approved by the Clinical Research Ethics Committee of Aizawa
Hospital (No. 2019-094).
Subjects and Methods
Subjects
Consecutive 29 patients with T2DM who took luseogliflozin
for 3 months or longer between January 1, 2019 to May 31, 2020
were initially registered. Because the purpose of this study was
to investigate the effect of luseogliflozin on NAFLD/NASH, 8 with
alanine aminotransferase (ALT) less than 20 IU/L [9], and other 2
with a habitual alcohol drinking of 20 g/day or more were excluded,
and the remaining 19 were analyzed.
The study was a single-arm, add-on study. Namely, 2.5
mg luseogliflozin was additively prescribed on top of the
hypoglycemic agents already taken by the patients, which are
shown in Supplemental Table 1. The data before and 12 weeks after
luseogliflozin administration was critically compared.
Laboratory measurements
In addition to the routine clinical chemistries, indices of the hepatic fibrosis including Fibrosis-4 index (Fib-4 index), gammaglutamyl trans peptidase (GGT) to platelet ratio (GPR-index), and aspartate aminotransferase (AST) to platelet ratio (APRI) were calculated10: the unit for AST, ALT, GGT as IU/L, platelet counts as 109/L and age as a year. Equations for each index were as follows [10].
Fib-4 = [(AST) ∙ (age)] / [(Platelet counts) ∙ (ALT)1/2]
GPR index = 100 ∙ (GGT) / (Platelet counts)
APRI = 100 ∙ (AST) / (Platelet counts)
Statistical analysis
The data were collected retrospectively and analyzed cross-sectionally and longitudinally. We evaluated the effect of luseogliflozin on the liver function tests and the indices of liver fibrosis. In addition, delta, i.e., the basal value minus 12 week-value for the indices of liver fibrosis was calculated for each study subject, and correlation between the delta values of fibrosis indices and the basal plasma glucose (PG), glycosylated hemoglobin (HbA1c), AST, ALT, GGT, body weight (BW) and body mass index (BMI) were examined. Furthermore, the delta value of the liver fibrosis indices and the delta value of PG, HbA1c, AST, ALT, GGT, BW and BMI were also examined. The statistical analysis was performed using JMP ver.15. Wilcoxon rank-sum test, Wilcoxon signed rank test and Spearman rank correlation were used as needed.
Literaturereview
Representative 10 original reports published in the English language on the treatment of patients with T2DM having NAFLD by SGLT2 inhibitors [6,7,11-18] were summarized to provide a current overview of the issue. In these literature, five kinds of SGLT2i agents were prescribed, with the number of the patients ranging from 9 to 32 and the treatment duration from 12 to 48 weeks.
Results
Baseline characteristics of the patients
Table 1. Baseline data (A), the data 3 months after the luseogliflozin treatment (B) and the difference (C) between the two (luseogliflozin- basal).
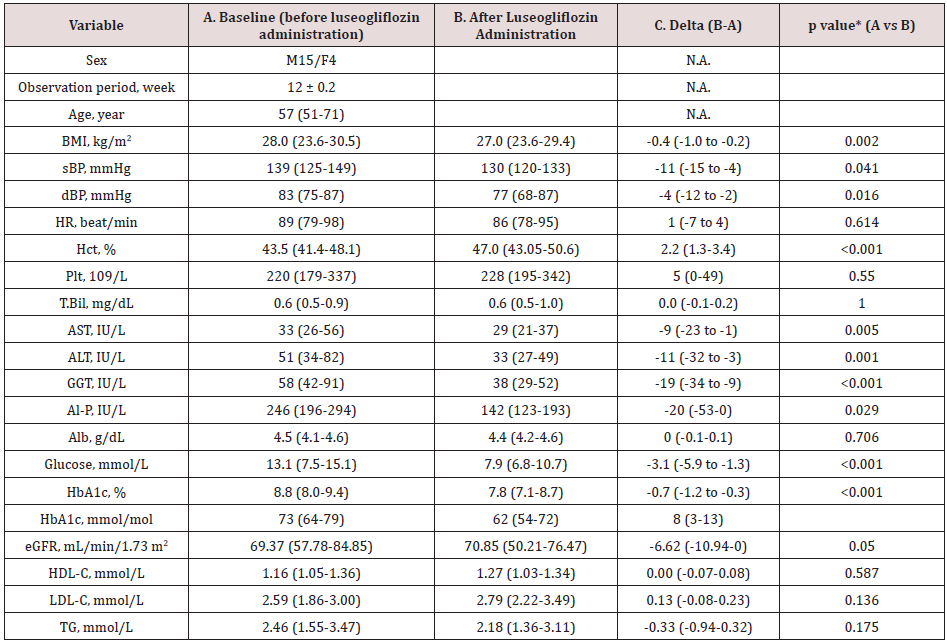
Values are median and interquartile ranges except for sex and the observation period: the latter was shown as mean and SD. sBP, systolic blood pressure; dBP, diastolic blood pressure; HR, heart rate; Hct, hematocrit; Plt, platelet count; T.Bil, total bilirubin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyl transpeptidase; Al-P, alkaline phosphatase; Alb, albumin; eGFR, estimated glomerular filtration rate; HDL-C, high density-lipoprotein cholesterol; LDL-C, low density-lipoprotein cholesterol; TG, triglycerides. N.A., not applicable. *Wilcoxon signed-rank test.
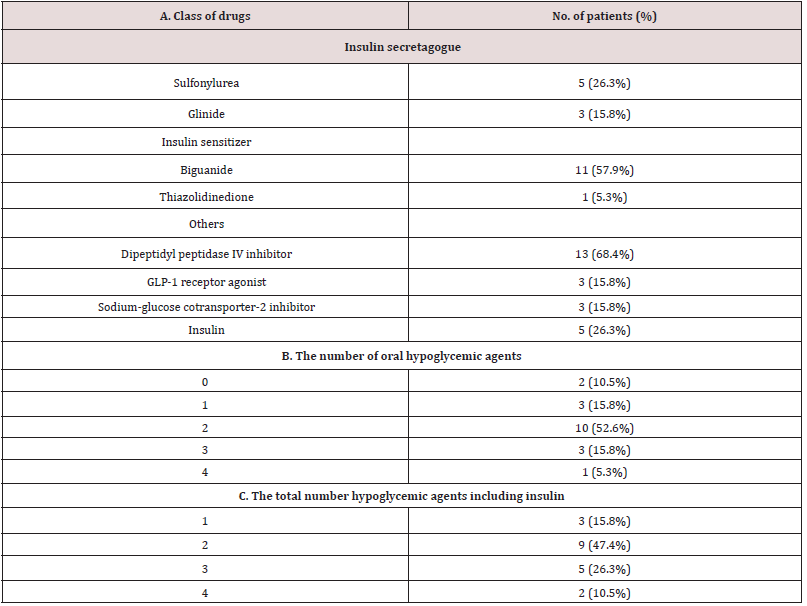
The study patients were male dominant (the proportion of males, 79%), middle-aged Japanese adults with the median BMI of 28.0 kg/m2 which was larger than the representative value in the Japanese patients with T2DM in general11(Table 1A). The median HbA1c value of the entire group before luseogliflozin was 8.8% (77 mmol/mol) so that the level of glycemic control was unsatisfactory (Table 1A). Regarding the hypoglycemic agents used before subscribing luseogliflozin, Dipeptidyl Peptidase-4 (DPP-4) inhibitors and biguanide were most frequently employed (Supplemental Table 1), which was typical for the Japanese patients [19].
Supplemental Table 1: Medications before administration of luseogliflozin. 2.5 mg luseogliflozin was added in each patient on top of the medication described above.
Values are median and interquartile ranges except for sex and the observation period: the latter was shown as mean and SD. sBP, systolic blood pressure; dBP, diastolic blood pressure; HR, heart rate; Hct, hematocrit; Plt, platelet count; T.Bil, total bilirubin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyl transpeptidase; Al-P, alkaline phosphatase; Alb, albumin; eGFR, estimated glomerular filtration rate; HDL-C, high density-lipoprotein cholesterol; LDL-C, low density-lipoprotein cholesterol; TG, triglycerides. N.A., not applicable. *Wilcoxon signed-rank test.
Liver function and indices of hepatic fibrosis following luseogliflozin administration
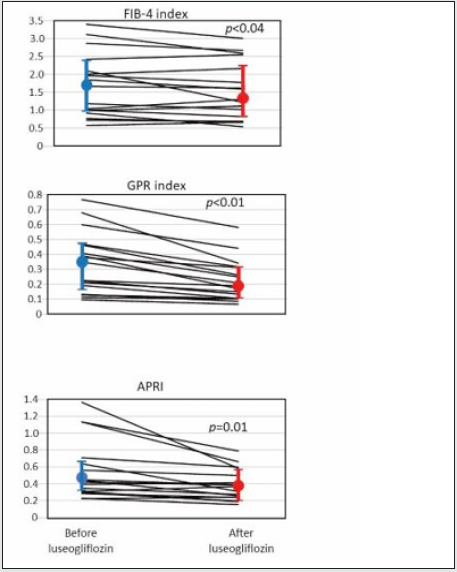
Elevated serum level of ALT was an inclusion criterion, so that the ALT was clearly elevated as a group with the median value, 51 IU/L. As well expected, administration of luseogliflozin significantly decreased PG and HbA1c (Table 1, A and B, before and after luseogliflozin, respectively). In addition, it significantly lowered the serum level of AST, ALT, GGT, and alkaline phosphatase (ALP). The degree of lowering was 12%, 34%, 35 and 42% for the respective enzyme levels. Importantly, the luseogliflozin treatment also significantly lowered the values for Fib-4 index, GPR index, and APRI (Figure 1).
Correlation between delta Fib-4, GPR index, and APRI and baseline and delta values of liver function and the body weight and BMI
Figure 1: Change of indices of liver fibrosis produced by luseogliflozin. Individual lines represent a change of the value in each person and the circles, and the vertical lines indicate the median and interquartile ranges: blue ones for before and red ones for after luseogliflozin. Wilcoxon signed-rank test was used for the statistical analysis.
The delta Fib-4 index was significantly and positively correlated
associated with higher baseline AST and ALT levels; the delta GPR
index was correlated with baseline AST and GGT; the greater delta
APRI was associated with higher baseline AST and ALT (Table
2A). Correlation between the delta values and the baseline of liver
function was absent for BW and BMI (Table 2A).
On the other hand, there was an inverse correlation between
‘delta Fib-4 and delta AST and delta ALT’, ‘delta GPR index and delta
GGT’ and ‘delta APRI with delta AST and delta ALT’ (Table 2B).
There was no significant correlation between delta values of the
fibrosis indices and the delta of BW and BMI (Table 2B).
Table 2. Correlation between delta Fib-4, GPR index and APRI and baseline value (A) and delta (B) of liver function. Delta means the difference between the value of each test performed on the day of starting luseoglifrozin and 12 weeks later.

Values are median and interquartile ranges except for sex and the observation period: the latter was shown as mean and SD. sBP, systolic blood pressure; dBP, diastolic blood pressure; HR, heart rate; Hct, hematocrit; Plt, platelet count; T.Bil, total bilirubin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyl transpeptidase; Al-P, alkaline phosphatase; Alb, albumin; eGFR, estimated glomerular filtration rate; HDL-C, high density-lipoprotein cholesterol; LDL-C, low density-lipoprotein cholesterol; TG, triglycerides. N.A., not applicable. *Wilcoxon signed-rank test.
Read More About Lupine Publishers Journal of Gastroenterology and Hepatology Please Click on Below Link: https://currenttrendsingastroenterology.blogspot.com/

No comments:
Post a Comment
Note: only a member of this blog may post a comment.