Lupine Publishers | Journal of Organic and Inorganic Chemical Sciences
Abstract
The titanatrane titanium complexes were treated with Aluminum Alkyls to prepare their bimetallic derivatives (1a-4a). The compounds were confirmed by means of NMR and elemental analysis. The complexes were used as catalysts for ring opening polymerization of racemic lactide. The complexes exhibit high activity and selectivity in the polymerization process.
Abbrevations: ROP: Ring Opening Polymerization; LA: Rac-lactide
Introduction
Olefin polymerization catalysis [1a-1b] continues to be an area of considerable importance to both the academic and industrial communities, and a wide range of reports are appearing on the efficient catalyst designs and application in various catalytic systems. Recently, the work on bimetallic complexes and in particularly bimetallic oxides is gaining considerable attention due to the "cooperativity" or "communication" between neighboring repeating units [2a-2d]. Since heterometallic alkoxides are potential molecular precursors of multicomponent oxides, they are thus of interest for applications in catalysis as well as in material science. Heterometallic alkoxide derivatives have been postulated to act as catalysts in Ziegler-Natta polymerization [3a,3b] or olefin metathesis reactions, [3c] and exhibit high activity and produce polymers with different microstructure. These heterometallic complexes were also found active in nitrogen activation," but detailed characterization is lacking [4].
The main disadvantage of mononuclear catalysts is the need of large amount of MAO or expensive fluorinated borate activators to obtain adequate polymerization activity, which causes concern over the high cost of metallocene catalysts and the high ash content (Al2O3) of the product polymers. Consequently, there is a great need to develop new catalyst systems that can provide high catalytic activity with no need for a large amount of expensive cocatalysts. We were thus interested in designing the catalysts which can exhibit high activity in the polymerization without or with very less amount of cocatalysts. For this, we used atrane ligands which have a nitrogen atom that facilitates coordination in a chelate fashion when necessary by providing the metal with additional electronic density [5a-e]. Although there have been many reports on the complexes based on atrane ligands, application of these complexes in polymerization reactions are very limited [6a-c].
We recently reported that heterobimetallic complexes of titanium iso-propoxide and aluminum alkyl containing bis (aryloxo) ethanolamine ligand were effective as catalysts precursor for ethylene polymerization even in the absence of cocatalysts [7]. We then extended the chemistry to tris (aryloxo) amine based ligands [8]. Furthermore, we reported that titanatranes bearing terminal substituted aryloxo ligands exhibit the highest activity in ethylene polymerization [9]. To the best of our knowledge these complexes are the best catalysts for ethylene polymerization among all the titanatranes reported so far. We became interested in isolating the bimetallic complexes of titatnium bearing aryloxo terminal ligands with aluminum alkyls to understand the plausible mechanism of polymerization process and the effect of substituents on the nature of heterobimetallic complexes. In this contribution we report the isolation, structural characterization of the titanatranes bearing aryloxo terminal ligand with the aluminum alkyls and their catalytic activity in ethylene and Ring Opening Polymerization (ROP) of rac- lactide.
Results and Discussion
The titanatranes and their bimetallic derivatives are prepared as reported earlier [8]. The following complexes were confirmed by NMR and elemental analysis. The pure crystalline products were used for the polymerization process (Figure 1). There has been considerable attention on the study of ring-opening polymerization (ROP) of cyclic esters such as rac-lactide (LA) with metal complexes for the past few decades [10a-b]. Various types of metal alkoxides such as tin [11a-e], aluminum, zinc , magnesium, iron [12a-b], lanthanide [13a-c], and lithium [14] organometallic complexes have been found to be active LA polymerization catalysts, and many afford materials with controlled molecular weights and narrow molecular weight distributions. Despite the fact that some excellent initiators have been reported for the polymerization of LA, the search for new catalysts that generate well-defined polylactides remains of keen interest. The roles of the structure of metal alkoxide complexes in determining molecular weights and molecular weight distributions, as well as the polymerization pathway, are significant current research issues.
Figure 1: Bulk Polymerization of rac-Lactide (rac-LA).
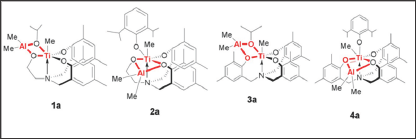
Recently, Verkade et al. [15a-b] reported that several titanium alkoxides showed reasonably good catalytic activity in the bulk homopolymerization of rac-LA at 130 °C. Harada et al. [16] also reported the living polymerization of LA by a Ti chloride complex, whose chloride apparently plays the same role as an alkoxide. We thus believed it would be interesting to test heterobimetallic titanium catalysts and compare the activity and control of the molecular and physical properties of the PLA produced by mononuclear and binuclear complexes with well-defined ligand environments. Here, we describe discrete heterobimetallic titanium alkoxide/aryloxide complexes and their bimetallic derivatives for the study of the ROP of LA under bulk polymerization conditions. We also demonstrate the difference in monometallic and bimetallic catalysts towards the ROP of rac-Lactide.
Preliminary results on the use of heterobimetallic catalysts for the bulk polymerization of rac-LA are summarized for 1a-4a, are presented in Table 1. Polymerizations were performed at 130 °C with the [LA]/[Ti] ratio fixed at 300. This table reveals that all the titanium compounds catalyze LA polymerization. Moreover, it appears that chelation aluminium methyl to the tripodal tetradentate ligand significantly decreases the polydispersity index and polymer yield. However, some transesterification probably occurred during the polymerization reaction since the polydispersity indices of both PLA products were somewhat higher than expected for a controlled polymerization. The bimetallic complexes exhibit high activity and produce polylactide with high molecular weight and lower polydispersity compare to their mononuclear precursors [15].
Table 1: Ring opening polymerization (ROP) of rac -Lactide Data for Heterobimetallic complexes.
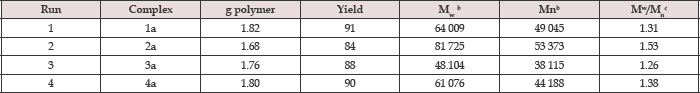
LA (2.027g) LA/Ti = 300, polymerization temperature = 130 °C, time = 20min.
The weight average molecular weight (M ), the number average molecular weight (M ), and the polydispersity index (PDI) M /M ) were determined by GPC.
Figure 2:

The preference for heterotacticity in our poly (rac-LA) are comparatively stronger for bimetallic complexes than their mononuclear precursor compounds and are similar to the previous reports by Kasperczyk et al. [17]. This may be due to the initiating alkoxide/aryloxide group which dissociate relatively easily from the titanium in bimetallic complexes than their monometallic precursors in the early stage of polymerization so that it can be utilized to initiate LA polymerization and provide a means of controlling the molecular weight by functioning as an end group. It appears that the initiating group is the highly bulky i-Pr alkoxide (in 1 and 3) or i-Pr-phenolate (2 and 4) group in monometallic, similar to the observation made by Verkade et al. [15] But the scenario in bimetallic complexes is complicated. We assume that the initiating group may be similar to the mononuclear complexes, although the insertion of lactide into Ti-O of the aryloxo arm or alkoxo arm cannot be ruled out (Figure 2) and (Table 1).
Concluding remarks
The titanatrane titanium complexes and their bimetallic derivatives exhibit high activity and selectivity in bulk polymerization of rac-Lactide. Bimetallic complexes (Ti-Al) exhibit higher activity and produces high molecular weight compared to their monometallic counterpart [5b]. This may be due to the better electronic and steric environment in bimetallic complexes. The polylactide obtained in this process is heterotactic in nature. Further investigations of mechanism in this process are on in our laboratory.
Experimental Section
General Procedures. All experimental manipulations were carried out under an atmosphere of dry nitrogen using standard Schlenk techniques or using a Vacuum Atmospheres drybox unless otherwise specified. All chemicals used were of reagent grades and were purified by standard purification procedures. Toluene (anhydrous grade, Kanto Kagaku Co., Ltd.) and n-octane (anhydrous grade, Aldrich) for polymerization were stored in a bottle in the drybox in the presence of molecular sieves (a mixture of 3A 1/16, 4A 1/8, and 13X 1/16). Polymerization grade ethylene (purity > 99.9%, Sumitomo Seika Co. Ltd.) was used as received. Toluene and AlMe3 from the commercially available methylaluminoxane [PMAO-S, 9.5wt% (Al) toluene solution, Tosoh Finechem Co.] were removed under reduced pressure (at ca. 50 °C for removing toluene and AlMe3 and then heated at >100 °C for 1 h for completion) in the drybox to give white solids. Bis (2-hydroxy-3,5-dimethylbenzyl) ethanolamine and tris(2-hydroxy-3,5-di-tert-butylbenzyl)amine were prepared according to a published procedure [18]. The titanatranes containing phenoxy terminal ligands Ti(O-2,6- iPr2C6H3){(O-2,4-Me2C6H2-6-CH2)2(OCH2CH2)N} and Ti(O-2,6- iPr2C6H3) [(O-2,4-Me2C6H2-6-CH2)3N] were prepared according to the previous report [9].
Molecular weights and molecular weight distributions for polyethylene were measured by gel permeation chromatography (Tosoh HLC- 8121GPC/HT) with a polystyrene gel column (TSK gel GMHHR-H HT x 2, 30cm *7.8mmΦ, ranging from <102 to <2.8x108 MW) at 140 °C using o-dichlorobenzene containing 0.05 w/v % 2,6-di-tert-butyl-p-cresol as eluent. The molecular weight was calculated by a standard procedure based on the calibration with standard polystyrene samples. All 1H and 13C NMR spectra were recorded on a JEOL JNMLA 400 spectrometer (399.65MHz for 1H, 100.626MHz for 13C). All deuterated NMR solvents were stored over molecular sieves under a nitrogen atmosphere in the drybox, and all chemical shifts are given in ppm and referenced to SiMe4 (TMS). All spectra were obtained in the solvent indicated at 25 °C unless otherwise specified. Elemental analyses were performed by using PE2400II Series (Perkin-Elmer Co.) [19].
Procedure for rac-Lactide Polymerization, LA bulk polymerizations were carried out by charging a stirring bar, 2.00g of LA, and then the appropriate amount of catalyst precursor to a 10ml Schlenk flask. The flask was then immersed in an oil bath at 130 °C, and after the appropriate time, the reaction was terminated by the addition of 5ml of methanol. The precipitated polymers were dissolved in a minimum amount of methylene chloride, and then, excess methanol was added. The resulting reprecipitated polymers were collected, washed with 50ml of methanol, and dried in vacuo at 50 °C for 12h [20a-b].
Read More About Lupine Publishers Journal of Chemical Sciences Please Click on Below Link:
https://lupinepublishers-chemicalsciences.blogspot.com/

No comments:
Post a Comment
Note: only a member of this blog may post a comment.