Lupine Publishers | Journal of Clinical & Community Medicine
Abstract
Aim: To study the use of nanotechnology in the diagnosis by the mean of breath and its treatment with nano medicine.
Material and Method: The study was carried out in 40 patients of different age groups and their breaths were exhaled. These breaths were analyzed by the use of sensors which were basically functionalized with gold nanoparticles. Sensitivity and specificity with relation to CKD patient classification according to estimated glomerular filtration rate were determined using cross-validation. The medicine was induced after the proper analysis of the target.
Results: It was found that the sensors were very useful when 2-3 nanoparticles were used together, and it showed that in between stage 1 and 2 CKD with an accuracy of 60% and in between stage 4 and stage 5 of CKD accuracy was 67%. There were various substances in breath which could identify many toxins which were accumulated due to kidney function loss. It was found that when treatments were provided using the nanomedicine the administration of the drug to target site was getting easy and many side effects due to drugs were avoided and was found to be a very useful method in the treatment of the patients with kidney diseases.
Conclusion: Nanotechnology in terms of both gold nanoparticles as well as nanomedicine has been proven to be a cost effective, reliable and a great source to target the tissues which need to be treated and shows a greater future potential in the diagnosis and treatment of kidney diseases.
Introduction
A novel process for identifying chronic kidney disease (CKD) and disease progression was explored that utilizes breath testing. CKD is a disease which causes the loss of kidney function from months to years and may lead to decreased kidney function [1]. It has been classified based on severity CKD in five stages according to the reduction in glomerular filtration rate (GFR), with stage 1 being a mild illness that is characterized by few symptoms and stage 5 being a severe illness that can be shown by a poor life expectancy if left untreated [2]. Untreated CKD progresses inevitably to stage 5, and various other procedures are applied in the patients with complete kidney loss like dialysis and kidney transplant this stage is also called as end stage kidney disease (ESKD) .up to 60% of the kidney function may be lost before serum creatinine begins to rise. These limitations and the onset of the disease without any symptom, contribute currently to delayed diagnosis and therapy of CKD. A conventional diagnostic technique that relies on the detection of volatile organic compounds (VOCs) among the plasma CKD biomarkers, or their metabolic products, are transmitted to the alveolar exhaled breath through exchange via the lung, even at the very onset of the disease, causing in later stages the fishy smell characterizing the breath of these patients. There is a very high progress in these coming years in the fields of nanotechnology and towards the standardization of breath sampling [3]. This could lead to the development of efficient and cost-effective methods for diagnostic breath testing that could eventually be introduced into standard medical practice, diagnostic imaging tests and conventional biomarker monitoring in blood and urine [4]. This shows that early detection of CKD and its disease progression helps in its treatment even in non-specialist treatment [5].
Haick et al. [6] had demonstrated the ability of an array of carbon-nanotube based sensors to differentiate between healthy states and induced ESRD in rats through breath samples, using a model of bilateral nephrectomy, and have achieved a success rate over 95% [1].
Nanotechnology involves the engineering of atoms and molecules at the submicron scale. The unique optical, electrical, physical, and chemical properties of matter at this scale can yield materials that behave differently from their macroscale counterparts [7]. This is referred to be as the most important and useful procedure to be used as it improves the pharmacokinetic and biodistribution and enables targeted delivery of drugs to specific tissues, cells, or sub cellular compartments. Additionally, achieving spatiotemporal, triggered, and controlled release of a variety of payloads (such as small-molecule drugs, contrast agents, peptides, proteins, deoxy and ribonucleic acids, and their combinations) over extended periods might be possible. Nanomedicines are typically degradable, biodegradable, and bio eliminable structures [8].
There are no clinically approved nanoparticles that specifically target the kidney for therapeutic or imaging applications. However, several recent pre-clinical studies describe nanomaterials that appear to selectively target renal tissues.
Several kidney diseases may benefit from the development of nanoparticle therapeutics that allow for the site-directed accumulation, controlled temporal release, and protection of a therapeutic payload [2]. Among candidate diseases are lupus, glomerulonephritis, and renal cell carcinoma (RCC), which often arises in the proximal tubules. Pharmacological therapeutic options for these diseases are limited, thus it is necessary to increase the efficacy and decrease side effects of current drugs. Irrespective of a molecular targeting element, nanoparticles may localize in one of several organs due to the particle surface chemistry, size, and zeta potential. The purposeful application of this mechanism may allow for the treatment of diseases regardless of the expression of molecular targets or the size of a lesion [9]. Nanomaterial size has a demonstrated effect on biodistribution. Certain synthetic polymers, low-molecular weight proteins, and peptides less than 20 kDa in molecular weight exhibit renal tubule biodistribution but are quickly cleared from the body.
This study mainly aims to the diagnosis of the disease in its early stages by using organically functionalized gold nanoparticles (GNPs).and to provide its treatment using the nanotechnology for the betterment of the patient before it becomes life threatening [3].
Material and Method
Study design: This study was a prospective study in which patients of the nephrology department of a secondary care hospital.
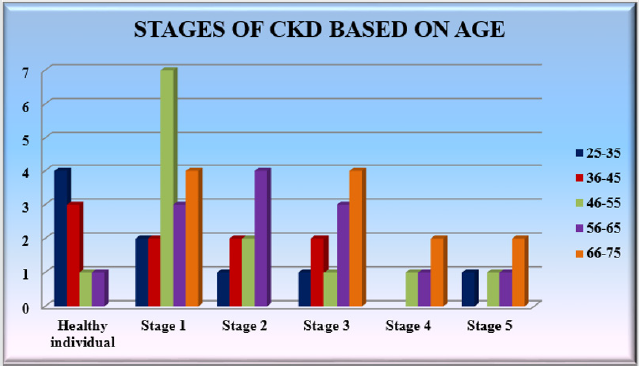
Study population: a total of 56 volunteer’s of age between 25-75 were randomly selected and their demographic details and previous reports were evaluated. There were 18 patients with stage 1 CKD, 20 patients who had intermediate CKD (stage 2-3), 9 patients with chronic CKD (stage 4-5) and 9 healthy individuals. They were undergoing with the treatment (calcium channel blockers, diuretics, angiotensin receptor blockers) (Table 1 & Graph 1).
Breathe collection and breathe analysis by using GNP sensors
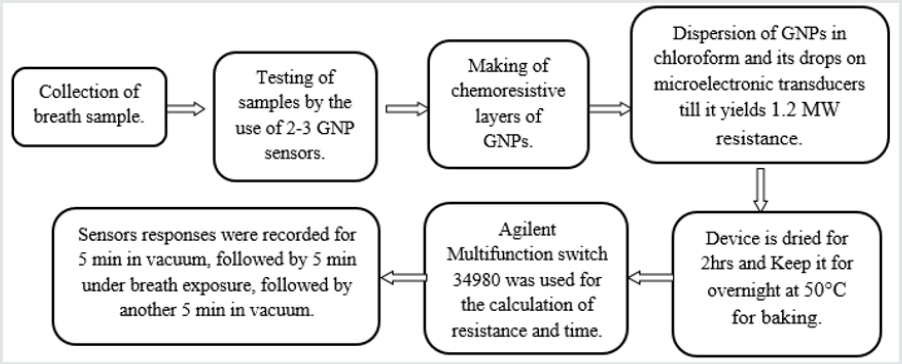
Alveolar breaths of patients were collected using an ‘offline’ procedure that effectively separates the endogenous from the exogenous breath volatile biomarkers and excluded the nasal entrainment [3]. The breath samples were tested with suitable combinations of two to three GNP sensors that were selected from a reservoir of 20 different GNP sensors developed by Haick and coworkers [1]. The sensors used were cross-reactive chemi resistors which were based on the four types of spherical GNPs with a core diameter of 3–4 nm. The organic ligands were: 2-ethylhexanethiol (S1), tert-dodecanethiol (S2), hexanethiol (S3), and dibutyl disulfide (S4). GNP chemi resistive layers which were formed by the dispersion of the GNPs in chloroform and then putting their drops on the microelectronic transducers. Then these were blown with dry nitrogen. This process was repeated several times to yield a resistance of approximately 1.2 MW. Then the device was dried for 2 hrs at a normal temperature and then it was put in a vacuum over night at 50 °C for the baking purpose. The microelectronic transducers had ten pairs of circular interdigitated gold electrodes that were deposited by an electron-beam evaporator TFDS-870. The outer diameter of this circular electrode was 3 mm and the width of each electrode and gap between each of them was 20μm. In total of 20 different GNP sensors were mounted on a custom polytetrafluoroethylene circuit board inside a stainless-steel test chamber with a volume of 100cm3. The sampling system delivered pulses of breath to the sensors and the chamber was evacuated between exposures. An Agilent Multifunction switch 34980 was used to measure the resistance. The sensors responses were recorded for 5min in vacuum, followed by 5min under breath exposure, followed by another 5min in vacuum. The cycles were repeated two- to three-times to test reproducibility (Figure 1).
Nanoparticle targeting of renal tissues
In particular there are no clinically proven nanoparticles that are used for the treatment of kidney diseases but some of them are being present that specifically target the renal tissues in 2 different forms.
Glomerular targeting
Various studies describe the targeting of nanoparticles to the glomerulus, portending the targeted treatment and imaging of this part of the kidney. Within the glomerulus, nanoparticles have been shown to target the glomerular basement membrane and mesangial cells. One study describes the localization of PEG-coated gold nanoparticles 80 nm in diameter in mesangial cells, although the majority of the particles accumulated in the liver. Follow-up work has suggested that similarly-sized nanoparticles transporting siRNA and composed of cationic polymers can accumulate and disassemble in the glomerular basement membrane. Nanoparticle surface charge appears to affect glomerular deposition. Cationic ferritin nanoparticles 13nm in diameter accumulated in the rat glomerular basement membrane, while negatively charged ferritin nanoparticles did not. Additionally, investigators have conjugated specific targeting agents such as antibodies to nanomaterials to increase their glomerular localization.
Tubular targeting
There have been few successful attempts to develop nanoparticles that target the renal tubules. One described strategy is the engineering of nanoparticles small enough to pass through the glomerular filtration barrier which could subsequently be absorbed by epithelial cells lining the lumen of the nephron. This strategy is similar to that employed for low-molecular-weight polymers and proteins.
A second strategy has employed biodegradable nanomaterials that pass through glomerular endothelial fenestrations but not the glomerular basement membrane. The hypothesized mechanism of uptake was degradation of the nanoparticle at this deposition site and subsequent megalin-mediated uptake of the degradation products by the luminal membrane of proximal tubular epithelial cells.
The authors of this review recently found selective targeting of the proximal tubules by large “mesoscale” nanoparticles which were much larger (~400nm) than the fenestration of the glomerular basement membrane. The authors hypothesized a mechanism whereby the nanoparticles transcytosis across peritubular capillaries to be endocytosed by proximal tubule epithelial cells from the basal side of the tubule. These nanoparticles localized in the kidneys with high specificity-up to seven times greater than any other organ.
Results
The choice of the GNP sensors for the identification of early-stage CKD
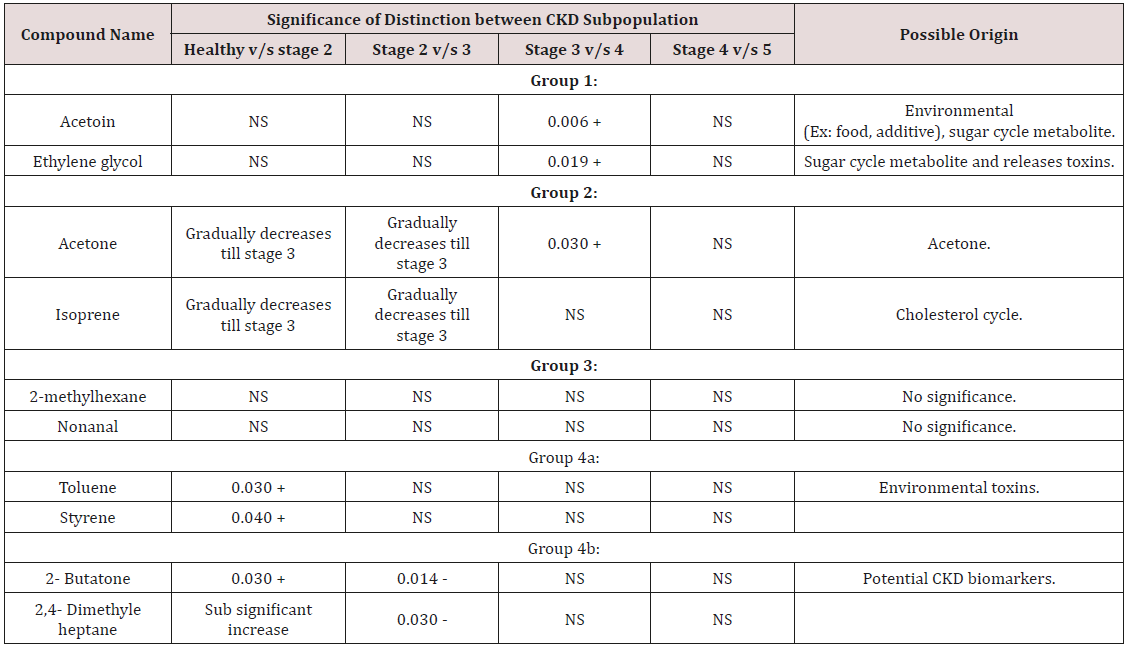
The feasibility of the GNP sensors for diagnosing the early stages of CKD was tested by comparing breath samples of 20 patients with stage 2 and 3 CKD to breath samples of 9 healthy controls (Table 1). Before the exposure to the breath samples, we examined the response of the GNP sensors to several representative VOCs found in exhaled breath. Each sensor underwent a rapid and fully reversible change in electrical resistance when exposed to the characteristic VOCs at concentrations ranging from tens of parts per billion to hundreds of parts per million, showing typical detection limits of 1-5 parts per billion [4]. The responses were unique because of the chemical diversity of the GNP ligands. The device-to-device variations between sensors based on the same type of GNP were within ± 15%, whereas the responses of different types of GNPs to the same VOC were typically several-fold different. So far, we have shown that part of the GNP sensors has a very low response to water. This is an important feature because otherwise, a sensor’s response to the high background humidity in exhaled breath could easily mask the signal to the much lower concentrations of the breath VOCs that indicate disease. The breath which is exhaled is mainly composed of nitrogen, oxygen, carbon dioxide, water vapor, and inert gases [10]. The VOCs that are generated by the cellular biochemical processes of the body are present in much lower amounts in exhaled breath, and many diseases, among them CKD, manifest themselves through very subtle changes in concentration of a multitude of these breath VOCs. The exposure of the GNP sensors to the breath samples resulted in rapid and fully reversible responses. Several sensing features were read out from the timedependent response of each sensor that related to the resistance response upon exposure (F1); the area under the response curve (F2); the sensor’s response time (F3) and the relaxation time after the end of the exposure (F4). Each sensor responded to all (or to a certain subset) of the VOCs found in the exhaled breath samples. However, the comparison between patients was based on compound masses and retention times. Three distinct trends were observed. The first group comprised isoprene and acetone, which are always present in exhaled breath in large quantities. Both substances decreased during stage 2 and remained relatively constant thereafter. Ethylene glycol and acetoin made up the second group of VOCs [11]. They remained relatively constant up to stage 3, showed a significant increase in stage 4 and a further, sub significant increase towards stage 5 [12]. The third group of compounds can be divided into two subgroups. Group 3a comprised the known industrial toxins styrene and toluene. Both compounds showed a significant increase during early-stage disease (stage 2), reached a plateau during stage 3 and remained relatively constant thereafter [13]. Group 3b contained two methylated hydrocarbons and a ketone, which showed a significant, brief increase during stage 2 CKD before their abundance decreased again to the levels observed in healthy controls. The fourth group comprised six compounds that were present in >75% of the CKD and healthy breath samples but showed no significant
Discussion
Identification of CKD & disease progression using the GNP sensors
It was done between the healthy individuals and diseased
individuals:
a) Healthy individuals with early stage CKD (I.e. stages 2 and
3)
b) Late-stage (stages 4 and 5) CKD.
(Table 2) differing it from the best feature set for the distinction of advanced and end-stage CKD. This can be understood if one considers the observed changes in the chemical composition of the breath during CKD disease progression [13]. However, the sensing mechanism of chem iresistive GNP layers is still the subject of scientific controversy. However, the organic ligands of the GNPs provide only a moderate chemical selectivity, so that the sensors are cross-reactive. The ligands are selected based on their ability to absorb certain (classes of) small VOCs that are typically found in exhaled breath as metabolic products. The changes in chemical composition between healthy and early-stage CKD differ from the changes in chemical composition between advanced and end-stage CKD [14]. Hence, distinct combinations of sensing features for the different chemistries would provide the optimal results in these two cases. This is because metabolic breath VOCs may appear at different concentration ratios in the breath of healthy persons, CKD patients, and is also different in individuals with different diseases.
The SVM classification revealed 73% sensitivity, 78% specificity, and 74% was the accuracy rate for the early-stage detection of CKD states. The obtained values fulfill the criteria for a good diagnostic method. The GNP breath test could be applied by general practitioners in non-specialist settings to ensure the speedy referral of a new patient to a specialist. Detecting progression from stage-4 to stage-5 is equally important because it usually marks the onset of dialysis treatment. The sensitivity, specificity, and accuracy of detecting the progression from early to late-stage disease using a single sensing feature were 75, 77 and 76%, respectively. Age, gender, diet, smoking habits, medication and exposure to hospital atmosphere are known to affect the chemical composition of the exhaled breath. Ideally, the study population should be matched in terms of these confounding factors. We have previously shown that the GNPs used by us have very little sensitivity to VOCs stemming from the above confounding factors. Shows that the population in the three studied cases was nevertheless relatively well-matched in terms of most of the confounding factors [1]. The compared groups are age-matched in the first two cases and gender-matched in the third case. Most of the volunteers were nonsmokers and the populations were well matched with respect for their smoking habits in all three cases. All CKD patients in this study were treated with calcium channel blockers, diuretics and/or a-blockers. The healthy controls did not receive these medications. Hence, the study population was matched in terms of medication in the last two cases. And then all the patients and the healthy controls were kept in the same hospital to give them the same environmental condition. Every other detail was also recorded because CKD patients suffer from various types of carbohydrate metabolism disorders, which could affect the breath concentrations of the VOCs in the first two groups. The majority of CKD patients suffer from glucose intolerance, which is mainly attributed to insulin resistance. Still, ethylene glycol could be of exogenous origin, because it is a known environmental toxin, and its accumulation during late-stage CKD could be attributed to the failure of kidney function. We assume that this is related to the continual death of kidneys nephrons, as up to 60% of the nephrons die before an accumulation of creatinine and urea could be observed. Styrene is an environmental toxin that causes DNA damage, and hydrocarbons are often released as environmental pollutants by the petrochemical industry the compounds in group 4 show no significant trends that could be related to the disease progression. The detected small molecules are VOCs that are either metabolic products or toxins that are absorbed from the environment and are accumulated in the body due to reduced kidney function. As such, each separate compound may appear not only in the breath of healthy persons but also in CKD patients. This way breathe samples of patients suffering from distinctly different diseases may have some common constituent compounds, but at different concentration ratios [15-16].
Conclusion
It concludes that there is a method in nanomedicine for detection of the early-stage CKD and monitoring disease progression from the exhaled breath of patients. Suitable combinations of GNP sensors can be used to identify the high accuracy patients with early-stage CKD and determine disease progression from advanced CKD to ESRD. The transition from early disease (stages 2 and 3) to advanced disease (stages 4 and 5) could be identified from a single sensing feature. Further in coming time we can use GNP sensors to distinguish with a higher resolution between CKD patients, so that the five stages of CKD progression can also be identified. For this purpose, a larger clinical trial will be conducted following this pilot study. GC-MS analysis identified three classes of VOCs in the breath that show distinctly different trends in average abundance during disease progression. The VOCs could be associated with accumulated environmental toxins or endogenous biomarkers of CKD-related medical conditions and body processes. A biomarkerbased breath test that utilizes GNP sensors could form the basis of a future cost-effective, fast and reliable (early) diagnostic test and monitoring tool for CKD. The nanoparticle field holds great potential for the diagnosis, imaging, and treatment of disease. An increasingly large body of pre-clinical work has demonstrated the potential for addressing renal diseases. In this review, we have outlined the applications of nanomedicines and highlighted recent work in the targeting of the kidneys. We also summarized the current state of the use of nanomaterials in renal disease treatment and diagnosis, and we pointed out several nephrological areas of need. We expect to see the increasing use of nanomaterials to fill technological gaps in the treatment and diagnosis of kidney diseases.
Read More About Lupine Publishers Journal of Clinical & Community Medicine Please Click on Below Link:
https://journalofclinicalcommunitymedicine.blogspot.com/


No comments:
Post a Comment
Note: only a member of this blog may post a comment.