Lupine Publishers | Journal of Organic and Inorganic Sciences
Abstract
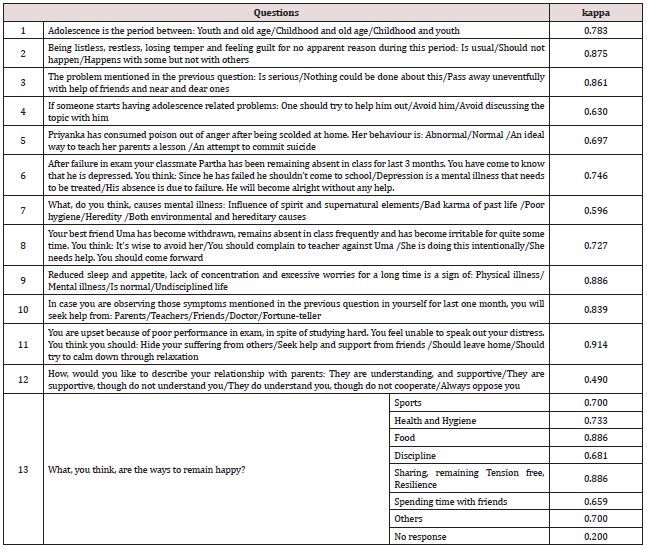
The Liquid Chromatography-Mass Spectrometry (LC-MS) is a powerful
analytical technique with very high sensitivity and specificity. LC-MS
is combination of Liquid Chromatography (LC) and Mass Spectrometry (MS).
With the Liquid Chromatography (LC) the separation of components can be
done and then the sample eluents from LC are transferred into Mass
Spectrometry (MS) where the detection, identification and determination
of masses of components can be done in presence of other components.
LC-MS is used in determination, of pharmaceutical drug substances,
intermediates and its related compounds for quantitative and qualitative
purpose. LC-MS is used most significantly in in-vitro dissolution,
bio-equivalence, bioavailability and metabolite studies. Also LC-MS is
used in basic research, agrochemical, forensic laboratories and food
industries .In this article principle of LC-MS, instrumentation and its
applications are briefly discussed.
Keywords: Liquid chromatography (LC); Mass
spectrometry (MS); High Performance Liquid Chromatography (HPLC); Liquid
Chromatography-Mass Spectrometry (LC-MS)
Introduction
Liquid Chromatography-Mass Spectrometry (LC-MS)
The High Performance Liquid chromatography (HPLC) is one of most
common analytical technique used in pharmaceutical industry for
determination and quantification of drug substances and its related
substances. Due to high reproducibility and accuracy, HPLC is routinely
used in pharmaceutical, chemical and pesticide industries.
The Liquid Chromatography-Mass Spectrometry (LC-MS) is hyphenated
analytical technique which is combination of Liquid Chromatography (LC)
and Mass Spectrometry (MS). HPLC (LC) separates the components of
mixtures by passing through chromatographic column. Generally, the
separated components cannot be positively identified LC alone. Mass
Spectrometry is also used for identification of unknown compounds, known
compounds and to elucidate the structure. Mass spectrometry is alone
not good for identifying mixtures because mass spectrum mixture is
actually complex of overlapping spectra from separated individual
components. It is difficult to connect Liquid chromatography (LC) with
Mass spectrometry (MS). An interface is used to transfer the liquid
eluents from LC to MS.LC-MS is more significantly used in invite
dissolution, bioavailability, bioequivalence and pharmacodynamics
studies [1]. Preparative LC-MS systems can be used for rapid
mass-directed purification of specific substances from such mixtures
that are important in basic research, pharmaceutical, agrochemical, food
and other industries [2,3].
Instrumentation
Liquid chromatography-mass spectrometry (LC-MS)
The Liquid Chromatography-Mass Spectrometry (LC-MS) is combination of
Liquid Chromatography and Mass Spectrometry which is used with
separation power of HPLC with detection power of Mass Spectrometry (MS).
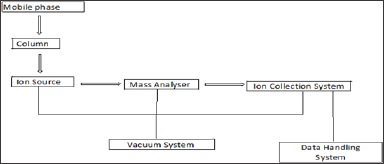
The schematic block diagram of LC-MS is shown in below figure 1. The
different parts of LC-MS instrument are listed as below.
a. Liquid Chromatography (LC)
b. Mass Spectrometry (MS)
Liquid Chromatography (HPLC): The Liquid Chromatography (LC)
is a high performance liquid chromatography in which separation of
components of mixture can be carried out by using liquid mobile and
solid stationary phase. There are different types of chromatography like
normal phase liquid chromatography, Reversed phase chromatography,
Ion-exchange liquid chromatography, Chiral separation and affinity
liquid chromatography [3]. By using different packing of columns with
high efficiency small amount of complex mixture can be separated. The
components of HPLC are listed below:
Figure 1: Schematic Block Diagram of LC-MS System.
a. Pump: It consists of material which is inert towards
solvents or any mixed composition of aqueous buffer and organic
solvents. It delivers high volume of mobile phase up to 10mL/min. There
are three major types of pumps are used i.e. reciprocating pump, Syringe
pumps and constant pressure pumps.
b. Sample injector: It is used to introduce sample volume into
the chromatographic system. Generally sample volume from 1|iL to 100|iL
can be injected. The injection volume can be increase by injector loop
up to 2mL volume. There are two major types of injectors used i.e.
Automatic injectors and Manual injectors. Automatic injectors are more
comfortable and user friendly and are more accurate and precise as
compare to manual injectors [3].
c. Columns: It is stationary phase which consists of silica
material in combination with carbon chain. Generally the column length
used is about 50mm to 300mm. The columns used in HPLC are consists of
Octadecyl (C18), Octyl (C8), Cyano, Amino, Phenyl packing's. The columns
are used on the basis of nature of compounds to be separated [4].
d. Detectors and recorder: The detectors is most important
part of HPLC .There are different types of detectors used are UV-Visible
detectors, PDA detectors, Refractive index (RI) detectors,
Electrochemical detector, Fluorescence detectors and conductivity
detectors. The signal received from detector can be recorded as peak and
respective data can be stored in a software.
Mass spectrometry: Mass Spectrometry is analytical technique
based on the measurement of the mass to charge ratio of ionic species
related to the analyte under the investigation.MS can be used to
determine the molecular mass and elemental composition of an analyte as
well as in depth structural elucidation of the analyte [5]. In LC-MS
there are two key components, ionization source and Interfaces. Below
listed are the different components of Mass spectrometers as below.
a. Ionization Sources and Interfaces
b. Mass Analysers
Ionization/Ion Source and Interfaces: The Liquid
chromatography separates mixture of components which are in liquid form,
usually contains methanol, acetonitrile and water. This liquid
containing mixture of components is transferred into the ion source of
mass spectrometer. As ion source is under high vacuum. Due to the
difference in the pressure it is difficult to mass to vaporize the
liquid drops without losing mixture of components. Hence interfaces are
used to resolve this problem. The different types of interfaces commonly
used in mass spectrometer are described as below.
a. Direct liquid Introduction (DLI): The ionization in Direct
Liquid Introduction (DLI) is generally accomplished by vaporizing
solvent as a chemical ionization and reagent gas. Both the normal and
reverse phase solvent system have been used. Reverse phase solvents used
are methanol/water, acetonitrile/ water mixture up to 60% water. In
general buffer with salts are not allowed as there is chance of
capillaries to plug when heated.
The operation of Direct Liquid Introduction (DLI) is combination of
thermal energy and liquid flow rate. The liquid enters the interface at
limited flow rate only. The analyte ions produced with the help of
thermal energy then transferred into ion source through capillary inlet
or pinhole diaphragm [6,7].
b. Atmospheric-Pressure Ionization (API): In
Atmospheric-pressure ionization (API) contains three major steps i.e.
Nebulisation, Evaporation and Ionization. There are two main modes of
API are Electrospray Ionization (ESI) and Atmospheric-pressure
ionization (APCI). In Atmospheric- pressure ionization (API), when
stream of liquid (solvent) containing a sample is passed through narrow
capillary tube and nebulized at large chamber, mist of small droplets is
produced .The ionization process takes place and the proportion of
droplets carry an excess of positive or negative electric charge .In
large heating chamber the evaporation of solvent takes place. The
solvent evaporates from the droplets to form smaller and smaller. The
collision takes place between the molecules and ions. The resulting ions
then passed through capillary into mass analyser [2,8].
The Atmospheric-pressure ionization (API) is technique used for wide
range of polar and non-polar analytes of moderate molecular weights.
c. Electrospray Ionization (ESI): The Electrospray Ionization
(ESI) is most useful ion source developed by Fenn and his colleague's.
In Electrospray Ionization (ESI) the liquid sample passed through a
stain steel capillary tube which is maintained at high positive or
negative electric potential about 3- 5kV [1]. Due to this the charged
droplets are formed at the capillary tip which are then undergoes
vaporization process. The solvent gets evaporated from droplets, and
undergoes reduction in size and surface charge increases. The collision
takes place until the highly charged droplets are converted into gas
phase ions. These gas-phase ions pass through the capillary sampling
orifice into the low pressure region of the ion source [9].
The major advantage of ESI is that the ions are multiply charged, the
number of charges increased by 1 to 3 for a molecule 1000Da or above
50000Da. This yields an m/z ratio that is always below 2000. LC-MS with
an Electro spray ionization (ESI) is used to measure the molecular
weight of peptides, Proteins, Biological samples, Polymers, nucleotides,
sugars and organometallics. It is also used frequently in Biological
research and medical analysis [10]. The Schematic diagram of ESI is
shown figure 2.
Figure 2: Electrospray ionization source.

d. Atmospheric Pressure Chemical Ionization (APCI): The
Atmospheric Pressure Chemical Ionization (APCI) include two major steps,
evaporation /desolvations of analytes and charged transfer reaction in
vapour phase to generate the vapour phase ions.
In Atmospheric Pressure Chemical Ionization (APCI) liquid (solvent)
containing sample is nebulised through narrow capillary tube and
nebulized into large chamber. In large heating chamber the evaporation
of solvent takes place at atmospheric pressure and small droplets are
produced. The ionization takes place. Generally ionization takes place
at 250 to 400 °C. The ions are then transfer the charges to molecules
through chemical reactions. The resulting ions are pass through
capillary orifice of mass analyser. It is widely used for less polar and
non-polar analytes having moderate molecular weights [11].
e. Thermo spray and Plasma spray Ionization (TSPI): The Thermo spray is used as both liquid inlet system as well as ionization source. Plasma spray is modification of thermo spray.
In Thermo spray the liquid sample solution is passed through
capillary tube which is heated and which causes the evaporation of
solvent. The charged droplets are formed. Due to evaporation of solvent
the droplets becomes smaller and smaller. The density of electric charge
on the surface of droplets increases. The resulting ions are then
passed into mass analyser with electrostatic voltage system [8].
The Plasma spray itself does not produce ions but the ions produce in
thermo spray, with the help of corona discharge or plasma the number of
ions can be increased. The electric discharge induces the more
ionization in the neutral molecules. This enhancement increases the
ionization of molecule. The plasma spray technique is more sensitive and
it is widely used for analysis in clinical and medicine [12].
f. Atmospheric pressure photo Ionization (APPI): In
Atmospheric pressure photo Ionization (APPI), photons are used to excite
and ionise the molecules. Atmospheric pressure photo Ionization (APPI)
include mainly two steps i.e. excitation and ionisation of analyte from
eluent.
Like atmospheric pressure chemical ionization (APCI) in Atmospheric
pressure photo Ionization (APPI) the eluent from LC vaporize into
gaseous phase. The APPI uses Kr lamp to produce photons. Kr lamp
generates high energy photons which are used for excitation and
ionization of molecules. The range of energy is selected to minimize the
ionization of analytes. The ionized analytes are then transferred into
capillary orifice into mass analyser (m/z).
This technique is useful for non-polar analytes which very much
difficult to ionize with Electrospray Ionization (ESI) and Atmospheric
Pressure Chemical Ionization (APCI) [13,14].
g. Particle Beam Ionization: The Browner and his colleagues
has developed particle beam interface to separate the solvent from
solute with minimum loss of solutes. The nebulization and evaporation
process are like Thermo spray (TSP), Atmospheric pressure chemical
ionization (APCI), Electrospray ionization (ESI) [15].
In this liquid separated from HPLC or LC, eluent is passed through
narrow tube. The liquid is injected with helium gas, due to this the
spray of liquid droplets are formed with high velocity. The liquid drops
from nebulizer passes through heating chamber, where the solvent begins
to evaporate and liquid droplets becomes smaller and smaller. The spray
of liquid droplets exits through heating chamber as a particle beam.
Then this beam passes through ionization chamber similarly like Electro
spray Ionization (ESI) and Atmospheric Pressure Chemical Ionization
(APCI) [2].
h. Continuous Flow Fast Atom Bombardment (FAB): The FAB is
simple, high sensitivity technique of interface. In FAB liquid target is
bombarded by fast atoms such as Argon(Ar) or xenon. The sample is
dissolved in glycerol and spread on thin layer metal plate /probe. Then
this probe is inserted into mass spectrometer and a beam of fast moving
atoms bombards on probe and ionise the samples which then pass into mass
analyser (m/z). FAB is used for large and thermally unstable molecules.
It used for surfactants and proteins [16,17].
Mass Analyser: After ionization the ions are transferred into
mass Analyser where the separation of ions are done according to their
mass to charge (m/z) ratio. Generally mass Analyser used is on its
speed, time, rate and its reaction.
Below are the mass Analyser:
a. Quadrupole
b. Time of flight
c. Ion trap
d. FTICR (Fourier transfer ion cyclotron resonance)
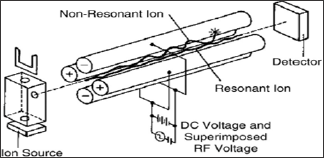
a. Quadrupole Mass Analyser: It is the most useful and
commonly used mass Analyser. It consists of two plain of parallel rods
which are located between an ion sources and a detector. The mass
Analyser i.e. separation of ion according to their m/z in either time or
space [6].
The linear Quadrupole mass Analyser consists of four hyperbolic or
cylindrical rods that are placed parallel in a radial array. Opposite
rods are charged is a +ve or -ve direct current(DC) potential at which
an oscillating radio frequency alternating current (RF) voltage is
superimposed [18].
The combination of DC and RF applied to the rods, trajectories of the
ions of one particular m/z are stable these ions are transmitted
towards detector. On the other hand ions of unstable m/z are discharged
on the rods.
The ions introduced into Quadrupole by mean of low accelerating
potential. The ions are oscillating in plane perpendicular to the rod
length as they trends through Quadruple filter.
Ions of carrying m/z consequently be travelled towards detector by
applying DC and RF voltage at constant ratio. The resolution depends on
ratio of DC and RF potentials. Generally the Quadrupole is operated at
<4000 m/z and scan speed up to 1000m/z passes. The unit mass
resolution means that mass accurately is seldom better than 0.1 m/z
[19].
The RF values are generally in the range 1-2MHZ. The DC Voltage may
be 1000V and Maximum RF voltage is 6000v. The schematic diagram of
quadruple mass analyser is shown below figure 3.
Figure 3: Schematic Diagram of Quadrupole Mass analyser. J
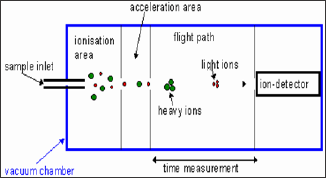
b. Time of Flight Analyser (TOF):
The time Flight is most robust used for wide variety of ions sources
and inlet systems. In this is no any magnetic field, maintenance and
calibration it is just simple electrostatic and straight forward. The
ions are extracted from source and subjected to an accelerating voltage.
The time taken to travel the length of the drift or flight to be
depends upon the mass of ion and its charge [20]. For single charged
ions (z=1, m/z =w) the time taken to reach the detector is proportional
to mass of the ions. When the ions trends towards the detector the
lighter ions will strikes the detector first [18].
Scanning of all the ions are detected simultaneously. The scanning
the mass range is very rapid and can be used for very large m/z values.
The schematic diagram of Time Flight mass analyser is shown below figure
4.
Figure 4: Time of flight mass Analyser.
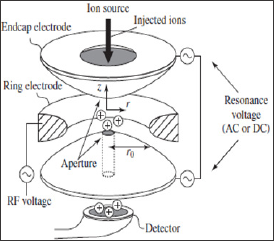
c. Ions trap mass Analyser:
Ion trap mass Analyser is high resolution, high sensitivity and multiple product ion scan capability.
A Quadrupole ion trap is a three dimensional ion trap. It consists of
cylindrical ring electrode to which Quadrupole field is applied.
Another two are end capped electrodes [21].
One end cap electrode has single small central aperture through which
electrons or ions are introduced into the trap while other one has
several apertures or holes through which ions are passed to a detector. A
Helium bath gas is present in the trap to stabilize the ion
trajectories. The collision takes place between helium bath gas and
ions. Due to this the motion of ions increases the trapping efficiency
of analyser. The ions are ejected from the trap on the basis of mass to
charge (m/z) values to create the mass spectrum [22]. The schematic
diagram shown below figure 5.
Figure 5: Ion Trap mass Analyser.
d. Fourier Transfer Ion Cyclotron Resonance (FT-ICR):
The Fourier transfer ion cyclotron resonance (FT-ICR) is most
important mass analyser. The ions arrived from ionization source are
passed into mass analyser where they are separated according to their
m/z ratio. The ions entered in chamber are trapped in circular orbits.
The ions are accelerated by both electric field and magnetic field.
Due to this the ions get excited and generate time dependent current.
The ions trapped separated according to mass to charge (m/z) ratios.
Detectors
The detector is an important tool of mass spectrometer that produces
the current that is proportional to the number of ions strike it. Once
the ions are formed passed from analyser they have to be detected and
transformed into signal. Below listed are the type of detectors commonly
used.
Point Ion Collectors Detector:
In this the ions collectors are placed at fixed point in mass
spectrometer. All the ions are focused upon the detector situated at
single point. The arrivals of ions can be recorded by the flow of
electric current and the data can be recorded. The electric current flow
is proportional to the ions arriving at point ion detector.
Array Detector:
An Array detector is collection of point collectors placed in plane.
The ions are arrived at a point or across the plane in array detector.
The ions with mass to charge (m/z) values are separated and are recorded
along plane using point ion collector. Spatially differentiated ions
with the mass range are detected simultaneously at the same time in
array detector [23,24] (Table 1).
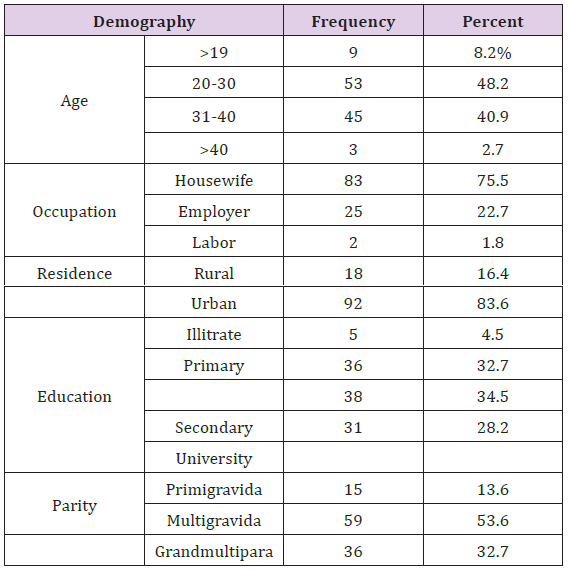
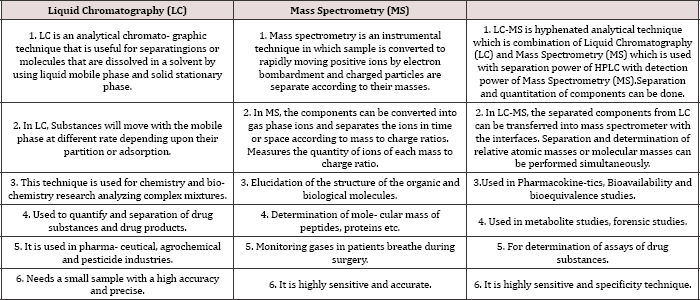
Table 1: Comparison between LC, MS and LC-MS.
Applications
Application of LC/ESI-MS in In forensic sciences
LC-MS is used for determination of toxicity, in drug analysis and
also in trace analysis. By using small amount of sample the toxins in
different material can be determined with LC-MS. Any toxic metabolites
in food or beverages can be determined by using LC-MS. E.g.
Identification of detergent added into orange juice can be determined by
analysing by the juice and detergent sample. The standard surfactant
alkyl diphenylether sulphonic acid is used. Both juice and detergent
samples are analysed in same chromatographic conditions. The mass
chromatograms and mass spectra obtained from the juice and detergent
samples are identical with the reference spectra of standard surfactant
(alkyl diphenyl ethersulphonic acid) [25,26] (Figure 6).
Figure 6: Extracted Ion Chromatogram of Orange Juice using LC-MS using ESI in negative mode.
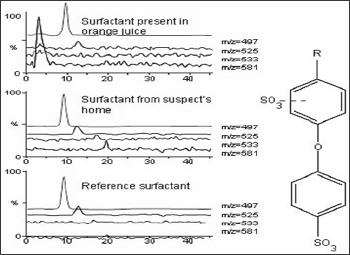
Figure 7: LC-MS Chromatogram in ESI (+) of Tuaminoheptane and
4-methyl-2-hexaneamine and mass spectra of 4-methyl-2- hexaneamine
which shows pseudo molecular ions [M+H]+ at m/z 116 yield the prominent
ion at m/z 57.
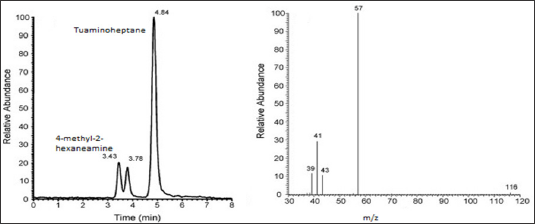
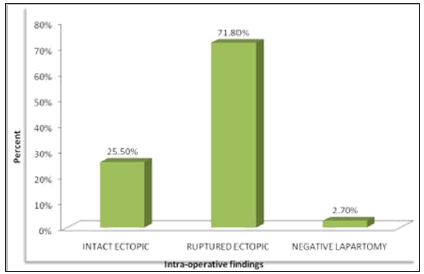
Application of LC-MS in Doping Test:
The LC/ESI-MS with positive mode can be used for detection in urine
of 4-Methyl-2-hexaneamine doping agent. The urine samples are analysed
with addition of internal standard of Tuaminoheptane. The suspected
primary amine 4-methyl-2-hexaneamine, an analog contained in dietary
supplement, to be the unknown compound. The standard used is
4-methyl-2-hexaneamine which exhibits two unresolved peaks at RT 3.43min
and 3.78min. which are identical with those of unknown compound [26].
The single reaction monitoring (m/z 116-57) was specific for detection
of 4-methyl-2- hexaneamine as shown below figure 7.
Other Applications:
In Pharmacokinetics:
LC-MS is used in the study of absorption, metabolism, and excretion
of drugs. Bio analytical methods are used for quantitative and
structural elucidation of drugs and its metabolites in the biological
samples (plasma, urine, saliva, serum etc.) [25].
In Bioavailability and Bioequivalence study:
Comparative bioequivalence studies in which quantitative
determination of drugs or metabolites is measured in biological matrix,
pharmacodynamics, clinical trials and In-vitro dissolution tests
[27,28].
In determination of molecular weights:
LC-MS is used for determination of molecular weights of known and
unknown compounds. It provides the information about molecular weight,
structure, identification, quantity of sample components. LC-MS is used
for determination of molecular masses of proteins, nucleic acids,
polymers and peptides.
In determination of Assay of drug and intermediates:
LC-MS is used in pharmaceutical industry for determination of assay
of drug substances, drug products, intermediates and their related
compounds [3].
In Agrochemical and pesticides industry:
It is used in determination of different components present in the fertilizers and pesticides [25].
Environmental Applications:
LC-MS is used for detection of phenyl urea herbicides, detection of low level of carbaryl in food [29].
Literature Survey:
Perrenoud L. developed LC-MS method for detection of 4-
methyl-2-hexaneamine a doping agent from urine.LC-MS with ESI in
positive mode is used. The analyte separated by gradient mobile phase on
reverse phase C8 column.The single reaction monitoring (m/z 116-57)
shows specific for detection of 4-methyl- 2-hexaneamine [26].
Allegrand J developed Atmospheric pressure photoionization (APPI)
mass spectrometry of guanine using tunable synchrotron VUV radiation. In
this APPI source coupled with tunable VUV photon source. The ionization
of guanine occurred by chemical reactions, as a function of photon
energy [13].
Pascual-Teresa Sd developed LC-MS method for analysis of anthocyanins
from from purple corn cob. The nine different types of anthocyanins are
isolated and identified by LC-MS. In this LC coupled with diode array
spectrometry and mass spectrometry and determined the anthocyanins
components from purple corn cob by fragmentation patterns (MS spectra)
[30].
Wang Y developed LC-MS method for analysing total resveratrol in
Grape juice, Cranberry juice and in wine. Samples were analysed by using
reverse phase HPLC with positive ion atmospheric pressure chemical
ionization (APCI) mass spectrometric detection. Resveratrol was detected
in grape juice, cranberry juice and in wine, the concentration ranges
from 1.56nmol/g, 1.07nmol/g and 8.63to 24.84 ^mol/L respectively [31].
Chang-Kee L describes the current developments in LC-MS for
pharmaceutical analysis. In this techniques like electrospray,
atmospheric chemical ionization and photo ionization and their
interfaces are discussed. The LC-MS application in drug discovery, in
vitro and in vivo drug metabolism, identification, characterization of
impurities in pharmaceutical analysis has been briefly discussed [1].
Nishikawa M reported the determination of surfactants by using LC-MS
in forensic toxicity. The analysis of anionic, cationic and non-ionic
surfactants are done in both negative and positive mode i.e. anionic
surfactants and positive surfactants are detected as M- ions in the
negative mode and M+ ions in positive mode while non-ionic surfactants
are in [M+H]+ ions or [M+NH4]+ ions in positive mode. The recovery range
obtained for anionic, cationic and non-ionic surfactants are 65.8% to
124 % [32].
Hernando MD, determined the trace level of pharmaceutical residues in
natural and treated water by using LC-MS. The samples like influent and
effluent wastewaters, rivers and tap waters are analysed. The
pharmaceuticals like Ibuprofen, Ketoprofen, and Diclofenac are
determined at trace level by using solid -phase extraction (SPE) with
liquid chromatography tandem mass spectrometry. The method detection
limit and quantitation limit were 7.5-75 ng/L [33].
Souverain S developed the method for determination of protein
precipitation for analysis of drug cocktail in plasma by using
LC-ESI-MS. For protein precipitation (PP), Acetonitrile (ACN),
perchloric acid (PA) and trichloroacetic acid (TCA) are used. The
LC-ESI-MS method was developed for simultaneous analysis of six tested
compounds in less than 6 minutes. Depending on the effective protein
precipitation techniques to remove protein from human plasma and
compatibility with LC-ESI-MS, ACN is used as PP technique in which
recovery above 80% and CV up to 6% obtained [34].
Bogusz MJ , developed the LC-ESI-MS method for detection of synthetic
adulterants in herbal remedies. Methanol is used for extraction of
drugs and are separated by liquid chromatography with gradient mobile
phase, Acetonitrile-10mM ammonium formate buffer pH 3.0. The limit of
detection reported is 5 pg to 1 ng per injection of sample. The
recoveries of spiked drugs obtained are from 63 to 100% [35].
Roach AGS, describes the application of LC-MS/MS method for analysis
for Acrylamide in foods. Acrylamide is used as internal standard. The
limit of quantitation [13 C3] is 10ppb (|ig/kg). The method is applied
to variety of foods. The variability in analyte level in food type helps
to decrease the acrylamide in foods [36].
Jangala H developed and validated the LC-MS method for simultaneous
estimation of Amlodipine and Valsartan in human plasma. The
chromatographic separation achieved by using isocratic method using
mobile phase as Acetonitrile: 5mM ammonium formate solution (80:20 v/v),
flow rate 0.8mL/ min. The quantitation was done using ESI in positive
mode under multiple reactions monitoring (MRM) condition. Assay obtained
in range 0.302-20.725ng/mL for Amlodipine and 6.062-18060.792 ng/mL)
for Valsartan [37].
Conclusion
The LC-MS is a hyphenated technique used in combination with
separation power of HPLC with detection power of Mass spectrometry. It
is widely used in pharmaceutical, chemical, food, agrochemical
industries, environmental and forensic applications. LC-MS is used for
qualitative and quantitative determination of drug substances and
biological samples. Also it is commonly used in drug research and
quality control.
Read More About Lupine Publishers Journal of Organic and Inorganic Sciences Please Click on Below Link:
https://lupinepublishers-chemicalsciences.blogspot.com/