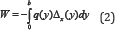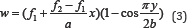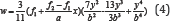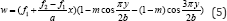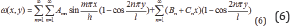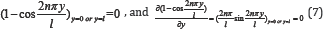Lupine Publishers| Journal of Drug Designing & Intellectual Properties
Abstract
Cambodian medicinal plants have been used to treat different diseases
such as cardiovascular diseases, inflammation, cancers, diabetes and
AIDS. This study aimed at conducting the Phytochemical analysis and in vitro antioxidant activities of whole plant of BryophyIIumpinnatum (Lam.) Kurz, barks of Dillenia ovata Wall. ex Hook. f. & Thomson, rhizomes of Drynariafortunei (Kunze ex Mett.) J. Sm. and barks of Lophopetalumwallichii
Kurz. native to Cambodia. All the plants were extracted with ethanolby
maceration extraction method. The Phytochemical analysis for alkaloids,
phenolic compounds, tannins, flavonoids, coumarins, steroids,
terpenoids, cardiac glycosides, essential oils, saponins and resins by
using the standard methods. The in vitro antioxidant property was
evaluated by assessing the DPPH' radical scavenging ability. The
preliminary Phytochemical evaluation of these species exhibited that the
ethanolic extracts of B. pinnatum (whole plant), D. ovata (barks), D.fortunei (rhizomes) and L.waIIichii
(barks) contain alkaloids, phenolic compounds, tannins, flavonoids,
terpenoids, cardiac glycosides, saponins and resins. Moreover, the
ethanolic extracts of B. pinnatum whole plant, D. ovata barks, and D.fortunei rhizomes possess coumarins and steroids, and of L.waIIichii barks have essential oils. The in vitro antioxidant activity of the species B. pinnatum whole plant, D. ovata barks, D.fortunei rhizomes and L.waIIichii barks have prominent antioxidant activities. This study suggests the potential source of natural antioxidant in B. pinnatum, D. ovata, D.fortunei and L.waIIichii native to Cambodia. Further research is highly recommended on the isolation of the antioxidant compounds from these species.
Keywords: B. pinnatum; D. ovate; D. fortune; L.waIIichii; Phytochemical analysis; In vitro antioxidant activity
Introduction
Reactive Oxygen Species (ROS) or free radicals are unstable
intermediates formed from molecules via the breakage of a chemical bond
such that each fragment keeps one electron, via cleavage of a radical to
give another radical and, also via redox reactions [1] . The ROS are
categorized into oxygen-centered radicals and oxygen-centered
non-radicals. The oxygen-centered radicals include superoxide anion (oO2_),
hydroxyl radical (oOH), alkoxyl radical (RO^) and peroxyl radical
(ROO^), and nitrogen species. The oxygen-centered non-radicals are
hydrogen peroxide (H2O2) and singlet oxygen (1O2), hypochlorous acid (HClO) and ozone (O3)
[2] . Free radicals are also generated either from normal essential
metabolic processes in the human body or from external sources such as
exposure to X-rays, ozone, cigarette smoking, air pollutants, and
industrial chemicals causing oxidative stress which is able to adversely
alter lipids, protein and DNA triggering various human complications
such as cancers, atherosclerosis, neurodegenerative diseases, diabetes,
age-related eye disease and Parkinson’s disease [3].
The free radicals can be scavenged by using the antioxidant system
including non-enzymatic constituents and a series of antioxidant
enzymes. The non-enzymatic constituents include glutathione, selenium,
vitamin C and E. The antioxidant enzymes embrace glutathione peroxidase,
catalase and superoxide dismutase which are the predominant antioxidant
enzymes playing a key role in minimizing the oxidative stress [4].
Several studies reported the free radical scavenging activities of
medicinal plants considered as the natural antioxidant, which is
utilized in several medical applications as their assurance of
effectiveness and safety [5]. The medicinal plants in form of roots,
barks, leaves, seeds and fruits have been used to manage various
illnesses since the ancient Cambodian era [6]. Medicinal plants are
composed of some bioactive compounds influencing the physiological
functions of the human body and these active phyto constituents include
alkaloids, terpenes and phenolic compounds [7], biosynthesized by the
pathways of acetyl coenzyme A, shikimic acid, mevalonic acid and
1-deoxylulose 5-phosphate in the plant [8]. The Phytochemical is being
broadly examined for its ability to provide health benefits, and several
reports proved their curative effects on cardiovascular diseases,
inflammation, cancers, diabetes and AIDS [9], the underlying mechanism
of which is due to their bioactivities as substrates for biochemical
reactions, cofactors of enzymatic reactions, and inhibitors of enzymatic
reactions [10].
However, no much scientific authentication has been made for most of
medicinal plants native to Cambodia. To address this lacuna, the present
investigation was carried out for the Phytochemical analysis and in vitro antioxidant activities of whole plant of BryophyIIumpinnatum(Lam.) Kurz (Crassulaceae) (local name: KabelLapoahs), barks of DiIIenia ovata Wall. ex Hook. f. & Thomson (Dilleniaceae) (local name: Phlu Thom), rhizomes of Drynariafortunei (Kunze ex Mett.) J. Sm. (Polypodiaceae) (local name: Baabrak), and barks of LophopetaIumwaIIichii Kurz (Celastraceae) (local name: Puen Ta Lei) [11].
Material and Methods
Collection of Plants
The whole plant of B. pinnatum, the barks of D. ovata, the rhizomes of D.fortunei and the barks of L.waIIichiiin
the dried form were collected from the local drugstore selling
medicinal plants in Phnom Penh, Cambodia, in August 2017. The plants
were authenticated with the voucher specimens UPFPT-110071 (B. pinnatum), UPFPT-110057 (D. ovata),
UPFPT-110069 (D. fortunei) and UPFPT-110066 (L. wallichii) of
University of Puthisastra (UP)- Herbarium. The parts of the plant
samples were deposited in the UP-Herm barium and the Pharmacognosy
Laboratory, Department of Pharmacy, Faculty of Health Sciences,
University of Puthisastra, Cambodia, aiming at conducting further
investigation.
Preparation of Plant Extracts
The maceration method was applied to the extraction of the plants.
Each plant part was powdered and kept in contact with ethanol in the
concentration of 157g/3500mL (B. pinnatum whole plant), 420g/6100 mL (D. ovata barks), 192g/3800mL (D.fortunei rhizomes), and 161g/5600mL (L.waIIichii
barks). The supernatants were collected by filtration after 24 hours,
and the solvent was evaporated to make the crude extracts. Each crude
extract was subjected to the lyophilisation. The residues obtained were
stored in airtight bottles in a refrigerator for the phytochemical
evaluation and the in vitro antioxidant activity. The plant
extraction was carried out at the Laboratory of Biological Pharmacology,
Department of Pharmaceutical Chemistry, Faculty of Pharmaceutical
Sciences, KhonKaen University, Thailand.
Phytochemical Analysis
The ethanolic extracts of B. pinnatum whole plant, D. ovata barks, D.fortunei rhizomes and L.waIIichii
barks underwent the Phytochemical screening in order to detect the
presence or the absence of alkaloids, phenolic compounds, tannins,
flavonoids, coumarins, steroids, terpenoids, cardiac glycosides,
essential oils, saponins and resins by using the standard methods [12].
This Phytochemical test was evaluated in the Pharmacognosy Laboratory,
University of Puthisastra.
Evaluation of Alkaloids (Dragendorff's, Mayer's and Wagner's Tests):
The crude extracts were dissolved with 1M-HCl (100mg/10 mL) and
subjected to the filtration. The filtrate was loaded equally into four
test tubes. One control test tube was added with no reagent, and the
rest of test tubes were treated with Dragendorff’s, Mayer’s or Wagner’s
reagents. The orange red (Dragendorff), creamy white (Mayer) or reddish
brown (Wagner) precipitates demonstrated the presence of Alkaloids [13].
Evaluation of Phenolic Compounds (Ferric Chloride Test): the
crude extracts were added with ethanol (100mg/10mL), and the solution
was filtrated. Two-milliliter filtrate was pipetted into the test tube,
following with the addition of distilled water 5mL. Four drops of
5%-FeCl3 were dripped into the filtrate. The formation of dark green precipitate showed the presence of Phenolic Compounds [14].
Evaluation of Tannins (Ferric Chloride Test): The ethanol was
added to the crude extract (100mg/10mL), and the mixture was filtrated.
The two-milliliter filtrate was transferred into the test tube and added
with few drops of 0.1%-FeCl3. The tested group was compared
with the control group, which was not added with the reagent. The
brownish green coloration interpreted the presence of Tannins [15].
Evaluation of Flavonoids (Ammonium Test): The crude extracts were dissolved in chloroform 2 mL and taken into the test tube. One milliliter of 1%-NH4
was added into it. The mixture was shaken vigorously. The yellow color
observed in the ammonia layer demonstrated the presence of Flavonoids
[16].
Evaluation of Coumarins (NaOH Test): the crude extracts with
the addition of ethanol in the concentration of 100mg/10mL were
filtrated. The two-milliliter of filtrate was loaded into the test tube
and added with 3mL of 10%-NaOH. The yellow coloration represented the
presence of Coumarins [17].
Evaluation of Steroids (Liebermann-Burchard Test): The crude
extract 100 mg was dissolved in the chloroform 2 mL and filtered into
the test tube. The mixture was added with 1 mL of glacial acetic acid,
followed by carefully the addition of 1ml of H2SO4 along the side of the test tube. The greenish color indicated the presence of Steroids [18].
Evaluation of Terpenoids (Salkowski's Test): The crude extract
of 100 mg was dissolved in the chloroform 5mL and filtered into the
test tube. The mixture was added carefully with 3mL of H2SO4
along the side of the test tube. The reddish brown color at the
interface of the two phases characterized the presence of Terpenoids
[19].
Evaluation of Cardiac Glycosides (Keller-Kiliani's Test): The glacial acetic acid 2 mL was mixed with 2 drops of 2%-FeCl3. The crude extract 100 mg was dissolved in this solution in the test tube. The mixture was added with 1 mL of H2SO4
along the side of the test tube. The brown ring at the interface
indicated the presence of Cardenolides, and the violet-green ring below
the brown ring in the acetic acid layer represented Glycoside. These
together characterized Cardiac Glycosides [19,20].
Evaluation of Essential Oils (NaOH-HCl Test): In the test
tube, the filtrate 2 mL of the extract was added with 100 μl of 1M-NaOH,
followed by the addition of 3 drops of 1M-HCl. The mixture was shaken.
The white precipitate demonstrated the presence of Essential Oils [21].
Evaluation of Saponins (Froth Test): the distilled water 15mL
were added to 100mg of the crude extract and filtered into the test
tube. The mixture was shaken for 10min until the formation of stable
persistent froth. Formation of stables five-minute persistent froth
indicated the presence of Saponins [22].
Evaluation of Resins (Turbidity Test): Ten milliliter of
distilled water were added to 200 mg of the crude extract and filtered
into the test tube, and the mixture was observed. The occurrence of
turbidity showed the presence of Resins [21].
DPPH' Radical Scavenging Assay: 1, 1-diphenyl-2-
picrylhydrazyl (DPPH) radical scavenging method is a rapid and sensitive
procedure to observe the antioxidant activity of plant extracts. The
stock solutions of the extracts were prepared in ethanol to achieve the
concentration of 1mg/ml. The dilutions were made to obtain the
concentrations of 100, 250, 500, 750 and 1000μg/mL (B. pinnatum whole plant); 2, 4, 6, 8 and 10μg/ mL (D. ovata barks); 150, 200, 250, 300 and 350μg/mL (D.fortunei rhizomes); and 20, 30, 40, 50 and 60μg/mL (L.wallichii
barks).The diluted solutions (100μL each) were mixed with 100μL of
ethanolic solution of DPPH (200μM). The mixture was left for 30min in
the darkness at room temperature, and the absorbance was recorded at
550nm. The experiment was replicated in three independent assays. The
solution without samples and with DPPH and ethanol was used as negative
control, and the Trolox (30μM) was used as a positive control. The
inhibition of DPPH free radical in percentage was calculated by the
formula:
Inhibition ( % ) = [( A negative control- A test )/(A negative control - A control)] x 100
Where Anegative control is the absorbance of the negative
control (DPPH + EtOH) and A is the absorbance of sample extracts. A
control is the absorbance of the control (Et OH alone). All tests were
run in triplicates (n=3), and average values were calculated [23]. The
assay was carried out in the Laboratory of Biological Pharmacology,
KhonKaen University. The IC50 parameter was used for the
interpretation of the results from DPPH method. The discoloration of
sample was plotted against the sample concentration in order to
calculate the IC50 value. It is defined as the amount of sample necessary to decrease the absorbance of DPPH by 50% [23].
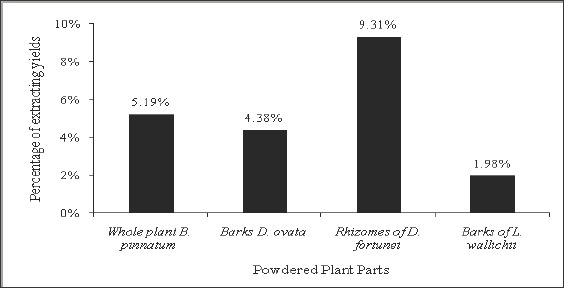
Figure 1: Ethanolic extracting yields (%) of four plant parts.
Results and Discussion
Extracting Yields (%) of Powdered Plants
The present study revealed that the ethanolic extracting yield of D.
fortunei rhizomes (9.31%) showed the highest value, following by the
yields of B. pinnatum whole plant (5.19%), D. ovata barks (4.38%) and L.waIIichii
barks (1.98%) successively (Figure 1). The maceration method is
considered as one of the general techniques of medicinal plant
extraction, and ethanol is widely used as a solvent to extract the
plant-derived chemicals [24]. Several reports showed the different
extracting values of different Cambodian plant extracts [25,26], which
is in consistence with our study indicating different percentages of
extracting yield. The technique of maceration extraction is commonly
used at the drug manufacturing enterprise line [27].
Preliminary Phytochemical Evaluation
The current study indicated that the ethanolic extracts of B. pinnatum whole plant, D. ovata
barks, D. fortune rhizomes contained alkaloids, phenolic compounds,
tannins, flavonoids, coumarins, steroids, terpenoids, cardiac
glycosides, saponins and resins, but showed the negative test of
essential oils. The ethanolic extract of L. wallichii barks demonstrated
the positive tests of alkaloids, phenolic compounds, tannins,
flavonoids, terpenoids, cardiac glycosides, essential oils, saponins and
resins; however, the coumarins and the steroids were not positively
tested (Table 1). Phytochemical are secondary metabolites that enable
plants to overcome temporary or continuous threats integral to their
environment, which is beneficial to the human in term of medical
treatment [28]. The plant-derived anticancerous drugs such asetoposide
and taxol have been applied for years in the clinical use and play a
crucial role in the development of the novel drug entities for human
[29]. The medicinal plants contributing towards the ethnomidicine have
been broadly screened for their PhytochemicalS including alkaloids,
tannins, saponins, steroid, terpenoid, flavonoids, phlobatannin and
cardic glycoside [30].They are associated with protection from and/or
treatment of chronic diseases such as heart disease, cancers, diabetes,
and hypertension as well as other medical conditions [31].
Table 1: Phytochemical screening of the ethanolic extracts of B. pinnatum whole plant, D. ovata barks, D. fortune rhizomes and L. wallichii barks.
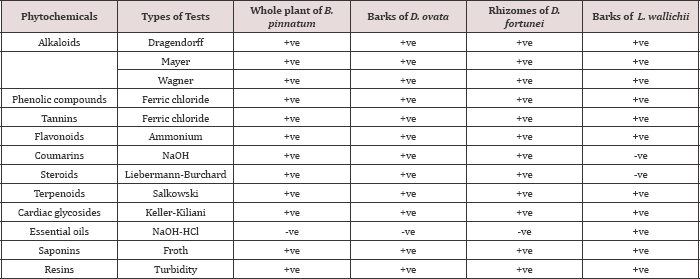
Note: +ve = Positive (present); -ve = Negative (absent)
DPPH Radical Scavenging Activity
The free radical scavenging activity of the ethanolic extract of
different plants was tested by DPPH radical method using Trolox as a
positive control. The DPPH assay provides information on the reactivity
of the test extracts with a stable free radical giving an absorption
band at 550nm [32]. The concentration of B. pinnatum whole plant ranged from 100-1000μg/mL, of D. ovata barks ranged from 2-10μg/mL, of D. fortunei rhizomes ranged from 150-350μg/ mL, and of L.waIIichii barks ranged from 20-60μg/mL. The ethanolic extract of B. pinnatum
whole plant inhibited the free radical in dose- dependent manner (R2 =
0.9068) with the inhibition percentage 17.72, 39.69, 65.35, 83.31 and
84.57% at the concentration of 100, 250, 500, 750 and 1000μg/mL
respectively (Figure 2). The ethanolic extract of D. ovata barks
inhibited the free radical in dose- dependent manner (R2=0.9361)with the
inhibition percentage 14.23, 32.77, 59.07, 60.35 and 75.06% at the
concentration of 2,4,6,8 and 10μg/mL respectively (Figure 3). The
ethanolic extract of D. fortunei rhizomes inhibited the free radical in
dose-dependent manner (R2 = 0.991)with the inhibition percentage 28.58,
34.6, 44.41, 50.36 and 56.23% at the concentration of 150, 200, 250, 300
and 350μg/mL respectively (Figure 4). The ethanolic extract of L.
wallichii barks inhibited the free radical in dose-dependent manner (R2 =
0.9648)with the inhibition percentage 27.41, 53.28, 60.21, 75.53 and
87.32% at the concentration of 20, 30, 40, 50 and 60μg/ mL respectively
(Figure 5). The IC50 of the ethanolic extracts of B. pinnatum whole plant,D. ovata
barks, D. fortunei rhizomes and L. wallichii barks accounted for
412.45, 6.23, 300.45, and 32.43μg/ mL respectively (Table 2). Several
studies reported the free radical scavenging potential of medicinal
plant extracts [33-35]. The antioxidant plant drugs play an important
role in the prevention and treatment of various disorders, caused by
oxidative stress, such as cancers, cardiovascular diseases, diabetes
mellitus, obesity and neurodegenerative diseases [36].
Read More About Lupine Publishers Journal of Drug Designing & Intellectual Properties Please Click on Below Link: https://lupine-publishers-drug-designing.blogspot.com/