Brass is an important metalloid which is used in construction of
sculptures. It is noticed that sculpture of brass is corroding due
to interaction of pollutants. The pollutants develop chemical and
electrochemical reaction on the surface of base material. Their
concentrations of corrosive pollutants are increased in winter season.
The air quality becomes very poor in winter season. Inside
sculpture different forms of corrosion are observed like galvanic,
pitting, stress, crevice etc. The major components of pollutants are
oxides of carbon, oxides of nitrogen, oxides of sulphur, ammonia, ozone
and particulates. Among these pollutants oxides of sulphur
and ammonia are major corroder of brass. Ammonia is observed moist air
to form ammonium hydroxide. It produces chemical
reaction with brass metal and form complex compounds like [Zn(NH
4)
4](OH)
2, [Zn(NH
4)
4]SO
4, [Zn(NH
4)]CO
3, [Cu(NH
4)
4](OH)
2,
[Cu(NH
4)
4]SO
4, [Cu(NH
4)]CO
3
etc. Oxides of sulphur react with moist air to exhibit sulphurous and
sulphuric acids. They interact
with brass to develop corrosion cell zinc metal and it is oxidized into
Zn2+ ions and these ions are active to humidity and carbon
dioxide to yield Zn(OH)
2.ZnCO
3.2H
2O. Copper is converted into Cu
2+ and it reacts with moist air and carbon dioxide to produce
Cu(OH)
2.Cu(CO
3)2 and these complex compound
detached on the surface of brass metal by rain water. These pollutants
change their
physical, chemical and mechanical properties and they also tarnish their
facial appearance. Brass’ sculpture is affected by uniform
corrosion. This type of corrosion can be control by nanocoating and
electrospray techniques. For this work (6Z)-5,8-dihydrazono-
5,8-dibenzo[a,c][8]annulene and TiO
2 are used as nanocoating and electrospray materials. The corrosion rate of material was
determined by gravimetric and potentiostat technique. The nanocoating and electrospray compounds are formed a composite
layer on surface of base metal. The formation of composite layer is analyzed by thermal parameters like activation energy, heat
of adsorption, free energy, enthalpy and entropy. These thermal parameters were calculated by Arrhenius, Langmuir isotherm
and transition state equations. Thermal parameters results are depicted that both materials are adhered with sculpture through
chemical bonding. The surface coverage area and coating efficiency indicates that nanocoating and electrospray are produced a
protective barrier in ammonia and sulphur dioxide atmosphere.
Keywords:Brass sculpture; Corrosion; Atmospheric pollutants; Nanocoating; Electrospray; Sulphur dioxide; Composite barrier
Introduction
The sculpture of brass comes in contact of contaminated air
thus its deterioration starts for protection various types methods
can be applied [1]. Brass [2] has major components is copper and
zinc. Zn reacts the hot air to produce ZnO which is active in humidity
[3] to convert into Zn(OH)
2. In moist air [4], they form CuO, ZnO,
Cu(OH)
2 and Zn(OH)
2. Both metals are active with sulphur to yield
Cu
2S, CuS and ZnS and these metallic sulphides [5] react with moist
air to give Cu(OH)
2, Zn(OH)
2, CuSO
4 and ZnSO
4. The hydroxides
of these metals interact with CO2 to produce CuCO
3 and ZnCO
3.
Sulphur dioxide [6] is a culprit of brass. It undergoes with Cu(OH)
2
and Zn(OH)
2 to convert into CuSO
4 and ZnSO
4. Moist SO
2 yields
H
2SO3 and H
2SO
4 whereas they create acidic environment [7] for
brass and generate corrosion cell on their surface. It accelerates
disintegration [8] in metal components of sculpture of brass. Brass
is highly sensitive to ambient of ammonia gas [9]. It interacts with
humid atmosphere [10] to NH4OH and it deposits on the surface
brass metal [11] thus it converts into a complex layer of [Cu(NH3)4]
(OH)
2 and [Zn(NH3)4](OH)
2 that layer erosion starts in rain water.
[Cu(NH3)4](OH)
2 and [Zn(NH3)4](OH)
2 complex compounds [12]
come in contact of H
2SO
4 environment to produce [Cu(NH3)4]SO
4
and [Zn(NH3)4]SO
4 that complex layer is eroded in rain water. In
acidic medium brass outer face has developed CuSO
4 and ZnSO
4 when
dust particulates [13] are deposited on their surface which contains
Fe to remove Cu and Zn from outer surface. Dust particulates are
possessed oxides of alkali metal in presence of moisture, it produces
NaOH or KOH [14] that is create hostile environment for Zn and it
forms complex compound [15] Na
2[Zn(OH)4]or Na[Zn(OH)
3.H
2O]
or Na[Zn(OH)
3.(H
2O)
3]. The oxides of NO2 reacts with moist air
to give HNO3 that acid produces chemical reaction with Cu and it
converted into Cu(NO3)2. Some organic acids [16] available in air
like acetic acid which develop corrosive environment for Cu and Zn
which converts Cu into Cu
2(CH
3COO)4.H
2O and Zn into (CH
3COO)
6.
Zn4O complex compounds [17]. They are eroded by rain water
on the surface of brass. Organic compounds [18] like amnio and
sulpur increased day by day in atmosphere. They develop hostile
environment for brass and corroding it. Corrosive pollutants [19]
concentrations like oxides of carbon, oxides of nitrogen, oxides of
sulphur, hydride of sulphur and nitrogen, ozone and particulates
are enhanced due to industrials wastes, effluents, flues and other
factors are like burning of coals, woods and cow dung cakes.
Harmful pollutants [20] come into atmosphere through agricultural
wastes, human wastes, pharmaceutical wastes, household wastes,
food wastes and decomposition of living things. Various types of
transports like road, water and air are evolving CO, NO2 and SO
2
gases which produce acidic environments for brass. Several types
of techniques are used to control the corrosion of brass like metallic
coating; polymeric coating, paint coating, organic and inorganic
coating of materials but these didn’t give satisfactory results in
corrosive medium. Some organic and inorganic inhibitors are
applied to protect the corrosion of materials in acidic but they
provide good results. Hot dipping, electroplating and galvanization
techniques is used as protective tools for brass corrosion in acidic
medium but these methods don’t shave base metals. In this work it
is to mitigate corrosion of brass corrosion by nanocoating and filler
techniques. These materials form composite barrier on the surface
base metal and blocked porosities and stop diffusion or osmosis
process of pollutants.
Experimental
Brass coupons 15sqcm were taken for experimental analysis.
Samples surface were rubbed with emery paper, rinsed with
acetone, dry them and kept into desiccators. Sample kept 20meter
height of roof in open sky and it observed that colour of brass can
be changed. Corrosion rate was determined in winter season by
weight loss method. The concentration of SO
2 in November 75ppm,
December 90ppm, January 105ppm and February 120ppm and
temperatures recorded in this period were 298K, 294K, 291K
and 295K. Synthesis organic compound (6Z)-5,8-dihydrazono-
5,8-dibenzo[a,c][8]annulene used as nanocoating and TiO
2 as
filler and corrosion of brass metal calculated in above mentioned
concentrations and temperatures in winter season. Both
compounds formed a composite barrier on surface of base metal
(Figures 1-4). Surface adsorption phenomenon studied by thermal
parameters like activation energy, heat of adsorption, free energy,
enthalpy and entropy.Potentiostat/Galvanostat model EG&G used
for corrosion potential, corrosion current and corrosion current
density. Brass sample put between H
2|Pt electrode as auxiliary
electrode and Hg
2Cl
2|HgCl
2 electrode reference electrode.
Figure 1: .
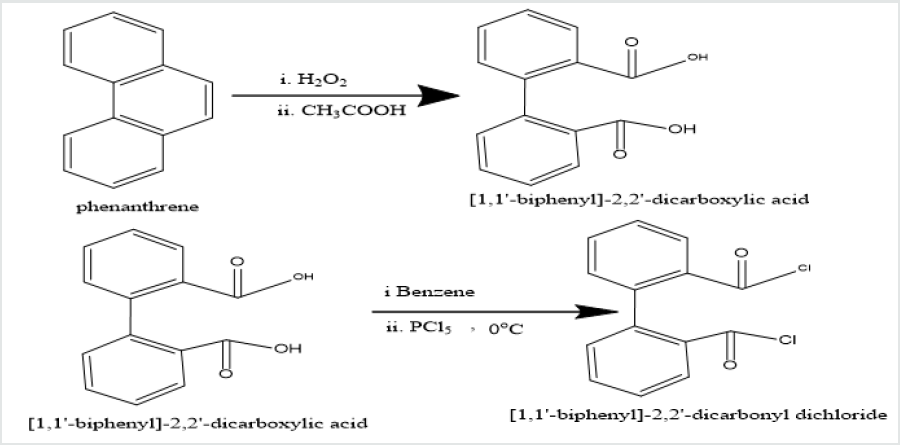
Figure 2:
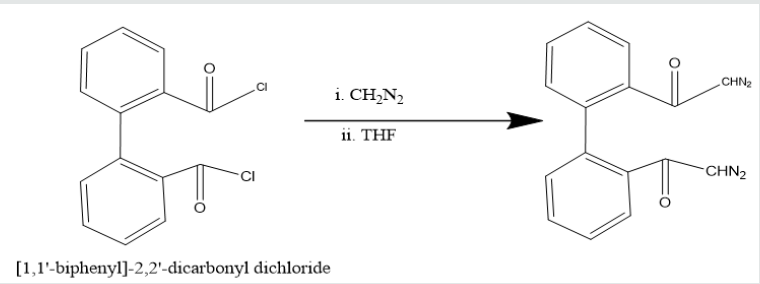
Figure 3:
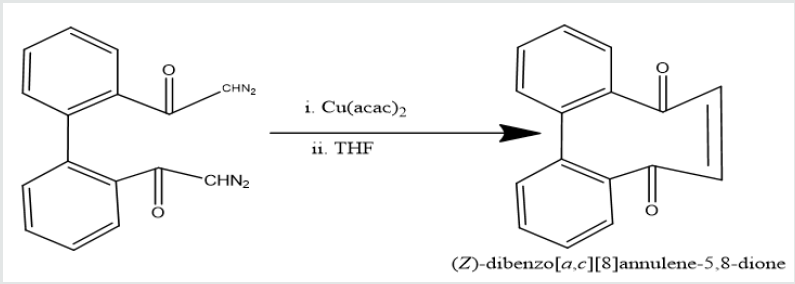
Figure 4:
Synthesis of (6Z)-5,8-dihydrazono-5,8-dibenzo[a,c][8]
annulene
Phenatharene was oxidized into [1,1’-biphenyl]-2,2’-
dicarboxylic acid by the use of H
2O2 in presence of CH
3COOH. When
[1,1’-biphenyl]-2,2’-dicarboxylic acid was treated in PCl
5 in benzene
solution at 0 oC temperature, [1,1’-biphenyi]-2,2’-dicarbonyl
chloride was obtained. It reacted with diazomethane to produce
yield [1,1’-biphenyl]-2,2’-dicarboxodiazomethan which heated
Cu(acac)
2 in presence THF to yield (Z)-dibenzo[a,c][8]annulene-
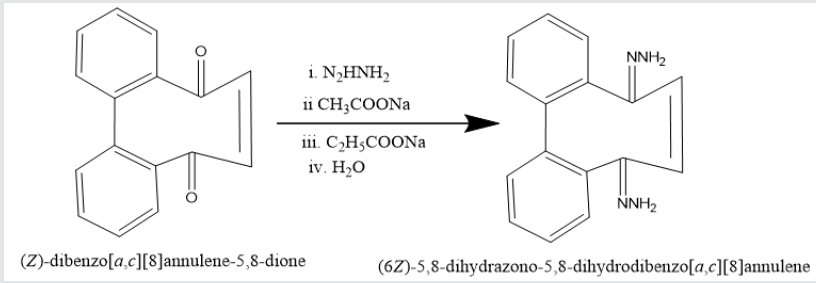
5,8-dione. It was used with hydrazine hydrate in ethyl alcohol to
give (6Z)-5,8-dihydrazone-5,8-dihydrodibenzo[a,c][8]annulene.
Results and Discussion
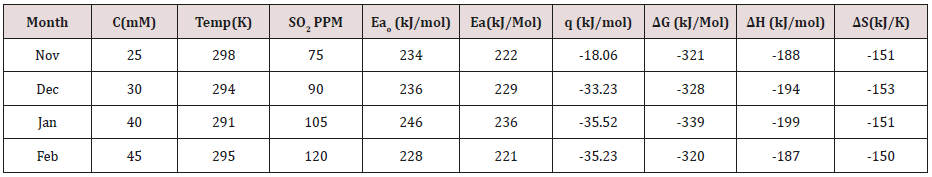
Brass metal was exposed in moist SO
2 environment in 75ppm,
90ppm, 105ppm and 120ppm concentrations and 298 0K, 294 0K,
291 0K and 295 0K temperatures. The corrosion rate of brass metal
was determined in winter season without coating and with coating
(6Z)-5,8-dihydrazone-5,8-dihydrodibenzo[a,c][8]annulene and
TiO
2 electrospray of by weight loss formula K= 534 W/DAT (where
W is weight loss, D is density and T is time) and their values were
mentioned in (Table 1)
Table 1:Corrosion of Brass Sculpture in Winter Season in SO
2 medium.
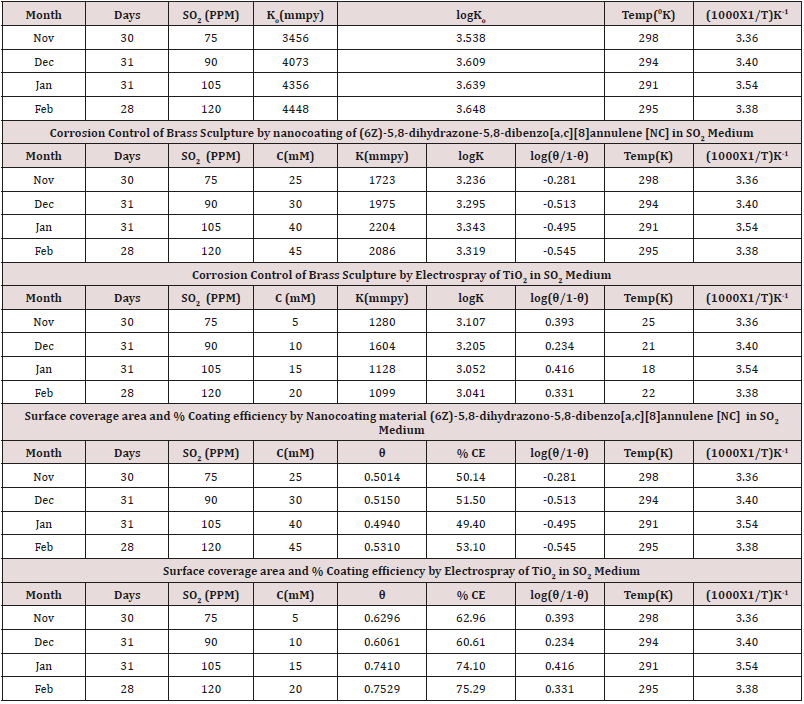
The corrosion rate of brass metal was recorded in the months
of November, December, January and February, the results (Table
1) was shown that corrosion rate of metal increased in January to
February but theses values were reduced with coating and filler
materials like (6Z)-5,8-dihydrazone-5,8-dihydrodibenzo[a,c][8]
annulene and TiO
2. It was clearly noticed in (Figure 5) K versus
Month. Brass metal kept into 75ppm, 90ppm, 105ppm and 120ppm
of SO
2 medium in month of Nov, Dec, Jan and Feb without coating.
It was coated with 25mM, 30mM, 40mM and 45mM concentrations
of (6Z)-5,8-dihydrazone-5,8-dibenzo[a,c][8]annulene, and again
kept into same concentrations of SO
2. After coating of (6Z)-5,
8-dihydrazone-5,8-dibenzo[a,c][8]annulene electrospray coating
of TiO
2 used at 5mM, 10mM, 15mm and 20mM concentrations and
same concentrations SO
2 Nov to Feb. The corrosion rates of in these
three cases were written in (Table 1). These results were shown
that corrosion rates without coating increased, it values decreased
coating with (6Z)-5, 8-dihydrazone-5,8-dibenzo[a,c][8]annulene
but their values more reduced with TiO
2 electrospray. These trends
were shown in (Figure 6) which plotted K versus C. The corrosion
rates of brass metal at different temperatures 298 0K, 294 0K, 291
0K and 295 0K without and with coating were recorded in (Table
1). The addition of nanocoating and electrospray were reduced the
corrosion rates as temperatures variation, it noticed in K versus T
in (Figure 7).
Figure 5: K(mmpy) Vs Months for brass metals.
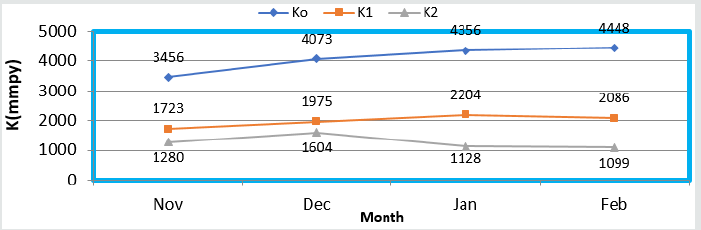
Figure 6: KVs T nanocoating and electrospray.
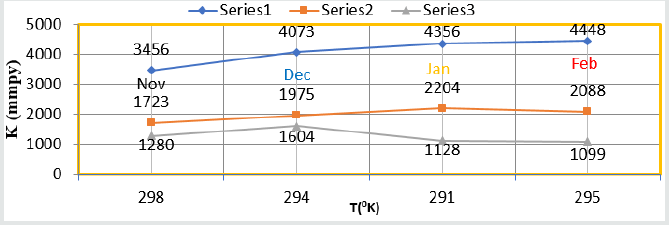
Figure 7: %CE Vs C(mM) nanocoating and electrospray.
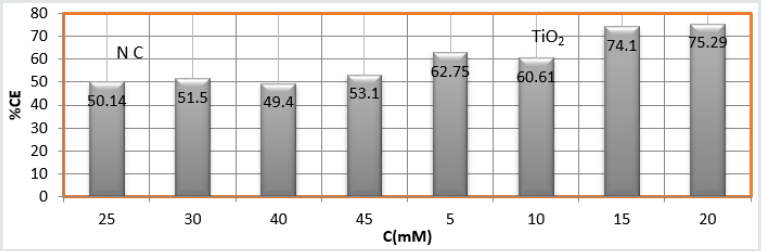
Figure 8: %CE Vs T for nanocoating and electrospray.
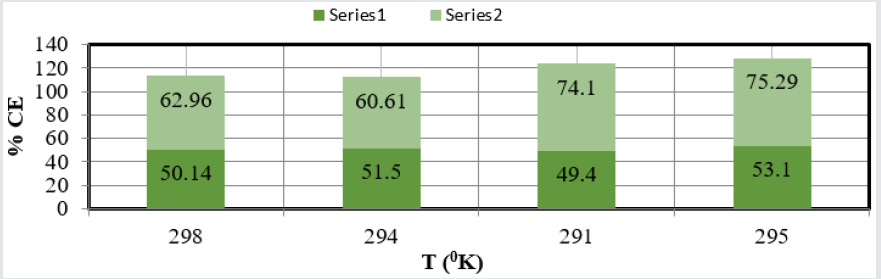
(Figure 8) plot between %C (percentage coating efficiency)
versus C (concentrations in mM) indicated that nanocoating
compound (6Z)-5, 8-dihydrazone-5,8-dibenzo[a,c][8]annulene
increased coating efficiency but TiO
2 electrospray produced more
coating efficiency with respect of nanocoating compound. The
values of % coating efficiency were calculated by formula %CE =
(1-K/Ko) X100 (where Ko is corrosion rate without coating and K is
corrosion rate with coating) and their values were given (Table 1).
(Figure 9) show plot between %C (percentage coating efficiency)
versus T (temperature in K). This figure indicated that percentage
coating efficiency enhanced as temperatures varies in Nov to Feb
months and their values were recorded in (Table 1). Figure 6
plotted between θ (surface coverage area) versus C (concentration
in mM) and covered areas were produced by (6Z)-5, 8-dihydrazone-
5,8-dibenzo[a,c][8]annulene and TiO
2 were mentioned in (Table 1).
The results were shown that nanocoating compound occupied less
surface areas with respect of electrospray. The surface coverage
area developed by nanocoating and electrospray compound was
calculated by formula θ = (1- K/Ko). (Figure 10) plotted between
θ (surface coverage area) versus T (temperature) noticed that
temperatures were varies from Nov to Dec but surface coverage
area and electrospray values were increased and their values were
written in (Table 1).
Figure 9: Vs C nanocoating and electrospray.
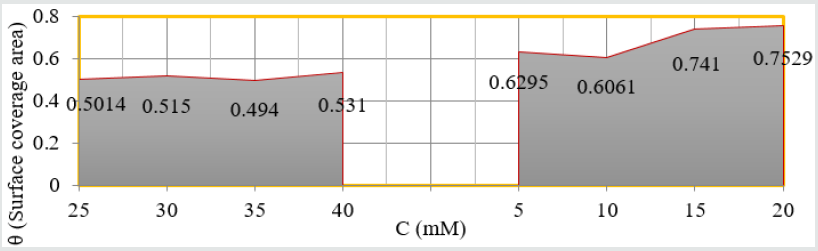
Figure 10: θ Vs T for nanocoating and electrospray.
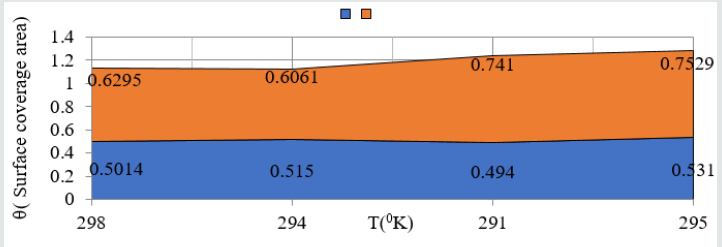
Table 2:Thermal Parameters of Brass Sculpture in Winter Season by Nanocoating of (6Z)-5,8-dihydrazono-5,8-dibenzo[a,c][8]
annulene [NC] in SO
2 Medium.
Composite surface formation was studied by Arrhenius
equation, Langmuir isotherm and others thermal parameters
like activation energy, heat of adsorption, free energy, enthalpy
and entropy and their values were recorded in (Table 2). (Table
2) Thermal Parameters of Brass Sculpture in Winter Season by
Nanocoating of (6Z)-5,8-dihydrazono-5,8-dibenzo[a,c][8]annulene
[NC] in SO
2 Medium. Activation energy of without coating, with
coating and electrospray coating were determined by Arrhenius
equation d(logK)/dT = A – Ea/2.303RT and their values were
recorded in (Table 2). The plot between logK versus 1/T was found
to be straight line as shown in (Figure 11). The plot between log
K and 1/T found to be straight line. It observed that activation
before coating activation energy high but decreased after coating.
These trends indicated that nanocoating compound adhered on
the surface of base metal. Heat of adsorption was calculated by
Langmuir isotherm log(θ/1-θ) = log(AC) – q/2.303R T and their
values were mentioned in (Table 2). Its values were found to
negative, it indicated nanocoating compound formed chemical
bond with base metal. (Figure 12) log(θ/1-θ) versus 1/T proved
results of heat of adsorption.Free energy values of nancoating
compound were determined by formula ΔG = -2.303 RT log(33.3K)
and their values were recorded in (Table 2). Their values found
to be negative; it noticed that nanocoating compound adhered on
the surface of base metal by chemical bond. Enthalpy and entropy
values of nanocoating and electrospray compounds were calculated
by transition state equation K=k T/N h e
ΔS/R e
-ΔE/RT and their values
were mentioned in (Table 2). These values were found to be negative
which indicated these compounds adhered on the surface of metals.
All thermal parameters versus T (temperature) plotted in (Figure
13) which indicated composite barrier formed on surface of base
metal. Thermal parameters Values of TiO
2 eleectrospray activation
energy, heat of adsorption, free energy, enthalpy and entropy were
written in (Table 3) and their plot against T (temperature) in (Figure
14). (Table 3) results indicated electrospray compound formed
chemical bond with nanocoating compound. (Table 3) Thermal
Parameters of Brass Sculpture in Winter Season by Electrospray of
TiO
2 in SO
2 Medium Potentiostat results were determined with help
of equation I = βa βc/2.3 (βa+βc) Ic and corrosion rate K=0.128
X Ic X( E/d) ( Ic is corrosion current, equivalent weight and d is
density) and their values were written in (Table 4). (Figure 15) was
plotted ΔE(corrosion potential versus I(corrosion current density).
The results of (Table 4) observed that without coating corrosion
potential high but with coating nanocoating and electrospray
reduced corrosion potential. (Table 4) Potentiostat results in SO
2 in
meduim with nanocoating and electrospray.
Figure 11: logK Vs 1/T nanocoating and electrospray.
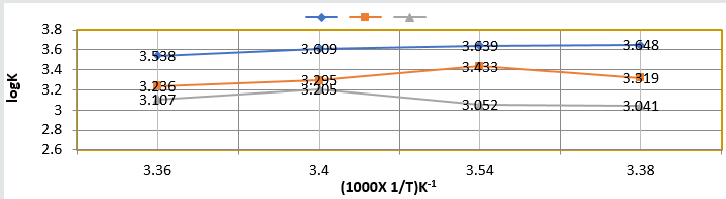
Figure 12: log(θ/1-θ) Vs 1/T nanocoating and electrospray.
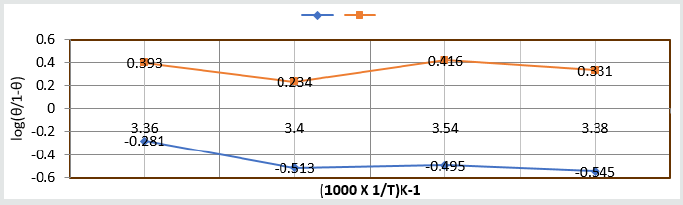
Figure 13: Thermal parameters Vs T for nanocoating and electrospray.
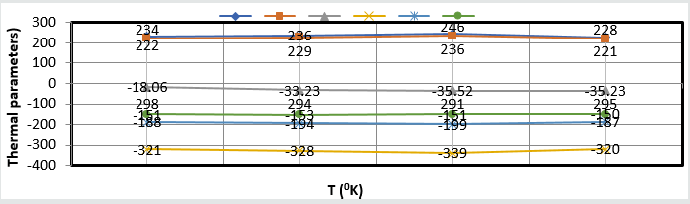
Figure 14: Thermal parameters Vs T nanocoating and electrospray.
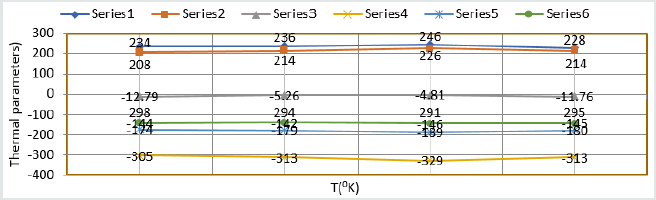
Table 3:Thermal Parameters of Brass Sculpture in Winter Season by Electrospray of TiO
2 in SO
2 Medium.
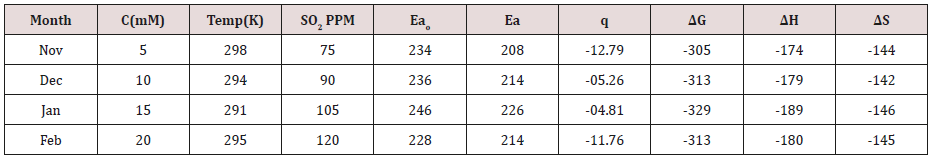
Table 4:Potentiostat results in SO
2 in meduim with nanocoating and electrospray.
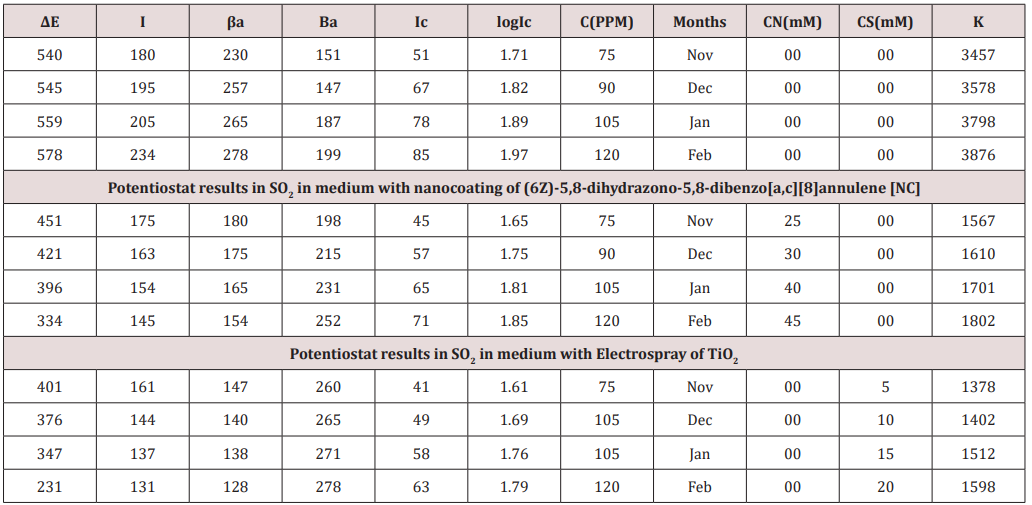
Figure 15: ΔE Vs Ic(mA) for nanocoating and electrospray.
Conclusion
It observed that winter season SO
2 concentration increased.
In this season humidity level found to more so it oxidized sulphur
dioxide into sulphuric acid and created hostile environment for
brass sculpture. It corroded zinc into zinc sulphate in longer period
it produced leaching corrosion. Such types of corrosion controlled
by the use of nanocoating of (6Z)-5,8-dihydrazono-5,8-dibenzo[a,c]
[8]annulene and TiO
2 electrospray. The results of activation energy,
heat of adsorption, free energy, enthalpy and entropy values
indicated that nanocoating compound adhered with chemical
bonding. Thermal parameters results of electrospray confirmed
that TiO
2 bonded with (6Z)-5,8-dihydrazono-5,8-dibenzo[a,c][8]
annulene chemical bonding. Both compound created composite
barrier on the surface of base metal which produced anticorrosive
barrier. The nanocoating compound developed lot of porosities
during coating. These porosities blocked by electrospray and it
increased coating efficiency and surface coverage area.
For more
Lupine Publishers Open Access Journals Please visit our website:
https://lupinepublishersgroup.com/
For more
Material science journal articles Please Click Here:
https://lupinepublishers.com/material-science-journal/
To Know More About
Open Access Journal Please Click on
Lupine Publishers






















No comments:
Post a Comment
Note: only a member of this blog may post a comment.